斑鳜(Siniperca scherzeri),属鲈形目(Perciformes)、  科(Serranidae)、鳜属(Siniperca)[1],为鸭绿江流域优质品种,因肉质细嫩、味道鲜美而深受广大消费者青睐。近年来,由于不合理的电鱼、毒鱼、过度捕捞和天然产卵场被破坏,其自然资源越来越少。目前,对鸭绿江下游斑鳜的人工繁殖[2⇓⇓⇓-6]、网箱养殖[7]、胚胎发育[8]及遗传学特征[9-10]等研究已有相关的报道,在营养价值分析方面,除了对比评价野生和养殖斑鳜肌肉营养成分和品质[11]外,还对比分析了其与同属鱼类之间的营养成分[12⇓⇓-15],但关于鸭绿江上游和下游斑鳜肌肉营养成分和品质评价方面的研究尚未见报道。近年来,有学者发现不同环境对经济鱼类的生长指标和营养成分影响较大[16⇓-18]。为此,本文对鸭绿江上游和下游斑鳜肌肉的营养成分进行分析,并对其脂肪酸营养价值作出初步评定,旨在为鸭绿江斑鳜的营养学、饲料开发及食用品质研究提供依据。
科(Serranidae)、鳜属(Siniperca)[1],为鸭绿江流域优质品种,因肉质细嫩、味道鲜美而深受广大消费者青睐。近年来,由于不合理的电鱼、毒鱼、过度捕捞和天然产卵场被破坏,其自然资源越来越少。目前,对鸭绿江下游斑鳜的人工繁殖[2⇓⇓⇓-6]、网箱养殖[7]、胚胎发育[8]及遗传学特征[9-10]等研究已有相关的报道,在营养价值分析方面,除了对比评价野生和养殖斑鳜肌肉营养成分和品质[11]外,还对比分析了其与同属鱼类之间的营养成分[12⇓⇓-15],但关于鸭绿江上游和下游斑鳜肌肉营养成分和品质评价方面的研究尚未见报道。近年来,有学者发现不同环境对经济鱼类的生长指标和营养成分影响较大[16⇓-18]。为此,本文对鸭绿江上游和下游斑鳜肌肉的营养成分进行分析,并对其脂肪酸营养价值作出初步评定,旨在为鸭绿江斑鳜的营养学、饲料开发及食用品质研究提供依据。
1 材料与方法
1.1 材料
2021年8月25日至2022年10月11日,分别从吉林省鸭绿江云峰段斑鳜茴鱼国家级水产种质资源保护区采集野生二龄斑鳜94尾(上游),从辽宁省丹东市爱河段收集野生二龄斑鳜48尾(下游)。
1.2 样品处理
活鱼测量完体长、体质量后,擦除表面水分,剥皮,取背部两侧全部肌肉,用滤纸将水吸干,再捣成肉糜,将样品放入-40 ℃冰箱中保存备用,在7 d内完成营养成分检测。
1.3 测定方法
1.3.1 常规营养成分测定
采用GB 5009.3—2016第一法(直接干燥法)测定水分;GB 5009.5—2016第一法(自动凯氏定氮法)测定粗蛋白;GB 5009.6—2016 第二法(酸水解法)测定粗脂肪;GB 5009.4—2016第一法测定粗灰分。
1.3.2 脂肪酸测定
测定方法参照GB 5009.168—2016第二法(外标法)。试样经水解—乙醚溶液提取其中的脂肪后,在碱性条件下皂化和甲酯化,生成脂肪酸甲酯,经毛细管柱气相色谱分析。气相色谱条件:色谱柱为30.00 m×0.25 mm×0.20 μm;进样口温度为 250 ℃;检测器(FID,260 ℃);色谱柱温度:140 ℃保持5 min,然后4 ℃/min升温,直至240 ℃后,保持10 min。载气:高纯N2;柱内流速19 cm3/s,分流比50∶1,标准混合脂肪酸甲酯及样品各自进样5 μL。在相同色谱条件下,依据标准脂肪酸的保留时间来确定脂肪酸组成。每个样品测定3个平行,取平均值。用校正峰面积归一化法计算其相对含量,选取含量较高的棕榈酸和油酸,进行加标回收率试验。平均加样回收率为81.0%~95.2%,RSD为3.45%~4.72%(表1)。由此可见,脂肪酸检测方法准确度高,仪器精密度良好。
表1 加标水平下棕榈酸和油酸的回收率及相对标准偏差
Tab.1
| 成分 Compound | 背景值/(mg/100g) Background value | 加标量/(mg/100g) Added content | 实测值/(mg/100g) Found | 回收率/% Recovery rate | 相对标准偏差/% RSD |
|---|---|---|---|---|---|
| 棕榈酸C16:0 | 15.8 | 1 | 16.61 | 81.0 | 3.45 |
| 2 | 17.59 | 89.5 | 3.96 | ||
| 5 | 20.38 | 91.6 | 4.72 | ||
| 油酸C18:1n9c | 19.7 | 1 | 20.58 | 88.0 | 3.93 |
| 2 | 21.55 | 92.5 | 4.69 | ||
| 5 | 24.46 | 95.2 | 4.05 |
1.4 数据处理
用Excel 2010和SPSS 19对数据进行统计和单因素方差分析,分析结果以平均值±标准差(Mean±SD)表示,组间差异则用Duncan’s检验进行多重比较分析,P<0.05为显著,P <0.01为极显著。
2 结果与分析
2.1 肌肉常规营养成分分析
鸭绿江上游和下游斑鳜的体质量、体长、水分、粗脂肪和灰分见表2。斑鳜体长为202~207 mm,体质量为175.33~196.52 g,年龄为2龄,上游个体明显大于下游个体。上游和下游斑鳜肌肉中水分、粗蛋白和灰分含量差别不大,上游斑鳜肌肉中粗脂肪含量显著高于下游斑鳜(P<0.05)。
表2 斑鳜鱼体的常规营养成分
Tab.2
| 种类 Speceies | 平均体质量/g Average body mass | 平均体长/mm Average body length | 水分/% Moisture | 粗蛋白/% Crude protein | 粗脂肪/% Crude fat | 粗灰分/% Crude Ash | |
|---|---|---|---|---|---|---|---|
| 上游斑鳜 S.scherzeri in upstream area | 196.52±22.59* | 207±2.45 | 76.5±2.70 | 19.1±0.52 | 2.0±0.16** | 1.12±0.01 | |
| 下游斑鳜 S.scherzeri in downstream area | 175.33±18.42 | 202±3.17 | 74.9±3.10 | 19.4±0.74 | 1.2±0.19 | 1.19±0.02 | |
注:表中数据为鲜重含量。统计分析采用的是单侧检验。**表示该指标在本组的平均值极显著高于另一组(P<0.01);*表示该指标在本组的平均值显著高于另一组(P<0.05);未标注的表示差异不显著。下同。
Notes: The data were the content in fresh weight.One sidedtest was used for statistical analysis.* * indicated the average value of this index in this group was very significantly higher than that of the other groups (P<0.01);* indicated the average value of this index in this group was significantly higher than that of the other groups (P<0.05);the difference between unlabeled representations was not significant(P>0.05).The same as below.
2.2 肌肉饱和脂肪酸、单不饱和脂肪酸及多不饱和脂肪酸含量
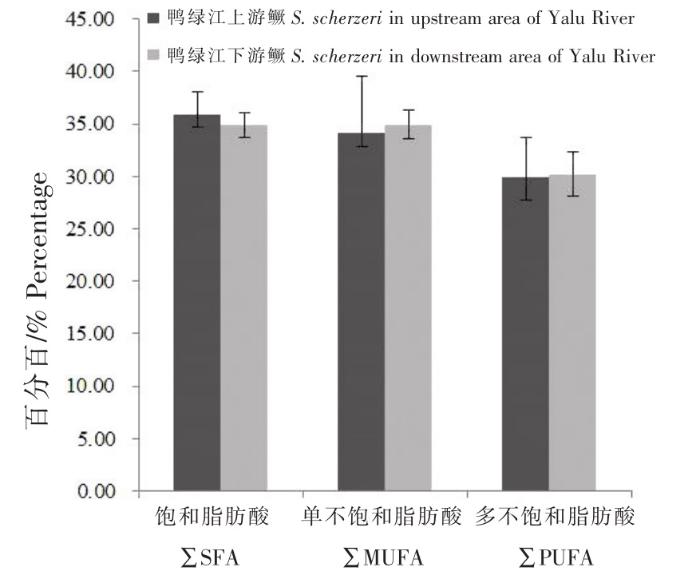
上游和下游斑鳜肌肉中的脂肪酸种类分别为16种和17种(表3),上游斑鳜肌肉中未检测到二十碳二烯酸(C20:2),但下游斑鳜肌肉中C20:2含量也较低,仅为1.632%;饱和脂肪酸(Saturated fatty acid,SFA)有6种,上游和下游斑鳜肌肉中SFA总含量分别为35.886%、34.913%,主要脂肪酸含量为棕榈酸(C16:0)>硬脂酸(C18:0)>二十碳酸(C20:0)>肉豆蔻酸(C14:0);单不饱和脂肪酸(Monounsaturated fatty acid,MUFA)3种,上游和下游斑鳜肌肉中MUFA总含量分别为34.184%、34.895%,C18:1>C16:1> C20:1;多不饱和脂肪酸(Polyunsaturated fatty acid,PUFA)8种,上游和下游斑鳜肌肉中PUFA总含量分别为29.922%、30.181%,其中主要脂肪酸为二十二碳六烯酸(C22:6n3,DHA)>亚油酸(C18:2n6c)>花生四烯酸(C20:4n6,ARA)>二十碳五烯酸(C20:5n3,EPA)>α-亚麻酸(C18:3n3)。由图1、表3知,斑鳜肌肉脂肪酸含量为∑SFA>∑MUFA>∑PUFA,但上游和下游斑鳜肌肉各总含量之间无显著性差异(P>0.05)。
表3 斑鳜脂肪酸组成及特征
Tab.3
| 脂肪酸 Fatty acids | 成分 Components | 鸭绿江上游斑鳜 S.scherzeri in upstream area of Yalu River | 鸭绿江下游斑鳜 S.scherzeri in downstream area of Yalu River | ||
|---|---|---|---|---|---|
| 饱和脂肪酸 SFA | 肉豆蔻酸C14:0 | 3.702±0.54* | 35.886±5.58 | 2.514±0.21 | 34.913±2.46 |
| 十五碳酸C15:0 | 0.529±0.08 | 0.455±0.07 | |||
| 棕榈酸C16:0 | 20.608±3.35 | 21.633±1.02 | |||
| 十七碳酸C17:0 | 0.790±0.14 | 0.670±0.09 | |||
| 硬脂酸C18:0 | 4.925±0.81 | 6.325±0.42* | |||
| 二十碳酸C20:0 | 5.332±0.61** | 3.316±0.49 | |||
| 单不饱和 脂肪酸 MUFA | 棕榈油酸C16:1 | 8.091±0.48 | 34.184±3.99 | 11.969±0.83** | 34.895±2.01 |
| 油酸C18:1n9c | 25.608±2.67* | 22.350±0.96 | |||
| 十碳一烯酸C20:1 | 0.485±0.09 | 0.576±0.04 | |||
| 多不饱和 脂肪酸 PUFA | 亚油酸C18:2n6c | 8.010±0.59* | 29.922±2.75 | 7.057±0.52 | 30.181±2.73 |
| γ-亚麻酸C18:3n6 | 0.292±0.05 | 0.245±0.08 | |||
| α-亚麻酸C18:3n3 | 3.725±0.31* | 2.514±0.12 | |||
| 二十碳二烯酸C20:2 | — | 1.632±0.06 | |||
| 花生四烯酸(ARA) C20:4n6 | 5.844±0.72 | 5.315±0.86 | |||
| 二十二碳二烯酸C22:2 | 0.379±0.06 | 0.479±0.04 | |||
| 二十碳五烯酸(EPA) C20:5n3 | 3.623±0.11 | 5.654±0.18** | |||
| 二十二碳六烯酸(DHA) C22:6n3 | 8.049±0.73* | 7.285±0.93 | |||
| 不饱和脂肪酸Unsaturated fatty acid | 64.106±6.25 | 65.076±4.79 | |||
| 必需脂肪酸EFA | 11.735±1.02* | 9.571±0.69 | |||
| DHA+EPA | 11.672±0.78 | 12.939±1.04 | |||
| ω-3系列多不饱和脂肪酸ω- 3 series of polyunsaturated fatty acids | 15.397±1.16** | 12.953±1.18 | |||
| ω-6系列多不饱和脂肪酸ω- 6 series of polyunsaturated fatty acids | 14.146±0.98** | 10.117±1.43 | |||
| ω-6/ω-3 | 0.919 | 0.781 | |||
图1
图1
饱和脂肪酸.单不饱和脂肪酸和多不饱和脂肪酸比较
Fig.1
Comparison of saturated fatty acids, monounsaturated fatty acids and polyunsaturated fatty acids
在SFA中,上游斑鳜肌肉的C14:0和C20:0显著高于下游斑鳜,而下游斑鳜肌肉的C18:0显著高于上游斑鳜(P<0.05)。在MUFA中,上游斑鳜肌肉的油酸(C18:1n9c)显著高于下游斑鳜,而下游斑鳜肌肉的棕榈油酸(C16:1)显著高于上游斑鳜(P<0.05)。在PUFA中,上游斑鳜肌肉的C18:2n6c、C18:3n3和DHA均高于下游斑鳜,而下游斑鳜肌肉的EPA显著高于上游斑鳜(P<0.05),上游和下游斑鳜肌肉中DHA+EPA总含量分别为11.672%和12.939%,下游斑鳜略高于上游,但差异不显著(P>0.05);上游斑鳜肌肉的必需脂肪酸(Essential fatty acids,EFA)、ω-3系列多不饱和脂肪酸和ω-6系列多不饱和脂肪酸含量均显著高于下游斑鳜(P<0.05),上游和下游斑鳜肌肉中ω-6/ω-3分别为0.919和0.781。
3 讨论
3.1 肌肉常规营养成分分析
通过对斑鳜肌肉常规营养成分测定发现,鸭绿江斑鳜的粗蛋白含量在19.1%~19.2%,且上游和下游差异不显著,但显著高于青石斑鱼(Epinephelus awoarp,15.37%)和褐点石斑鱼(E.fuscoguttatus,15.18%)[19];其粗脂肪含量在1.2%~2.0%,上游斑鳜粗脂肪含量(2.0%±0.16%)显著高于下游斑鳜(1.2%±0.19%),也显著高于大眼鳜(Siniperca kneri,1.15%)[15]、翘嘴鳜(Siniperca chuatsi,1.50%)[15]、黄颡鱼(Pelteobagrus fulvidraco,1.57%)[20]、野生大黄鱼(Pseudosciaena crocea,1.61%)[21] 和养殖马口鱼(Opsariichthys bidens of culturing,1.79%)[22],与草鱼(Ctenopharyngodon idellus,1.37%~2.25%)[23]接近。分析原因可能为:一是采样时间不同所致,从2021年8月25日至2022年10月11日,因采样条件受限,在鸭绿江上游和下游的每次采集样品都存在时间间隔,这会导致个体大小存在差异;二是由各自生境差异及其构成的食物链不同而致,鱼类摄入的饵料生物的种类、数量及比例直接影响鱼肉的营养价值[24],环境稳定性也是鱼体脂肪含量的关键因素,上游环境的不稳定性造成斑鳜需要贮备较多能量用于生存,主要体现在肌肉中脂肪的积累[25],水环境中的理化因子(盐度、水温和氧气等)对鱼类的品质存在显著影响[26-27]。有研究证实肉质风味、脂肪酸含量与肌肉脂肪含量成正比[28],因此推测上游斑鳜肉质风味和鲜味程度会更胜一筹。
3.2 肌肉脂肪酸营养评价
脂肪酸是脂肪的重要组成部分,是生物体内必不可少的营养成分之一[29]。鸭绿江上游和下游斑鳜肌肉中单不饱和脂肪酸总含量(34.184%、34.895%)均远高于湖鲚(Coilia nasus taihuensis,31.49%)[25],斑  (Hemiramphus far,20.60%)、南洋
(Hemiramphus far,20.60%)、南洋  (Hemiramphus lutkei,19.27%)、缘下
(Hemiramphus lutkei,19.27%)、缘下  (Hyporhamphus limbatus,26.26%)、瓜氏下
(Hyporhamphus limbatus,26.26%)、瓜氏下  (Hyporhamphus quoyi,16.28%)、叉尾鹤
(Hyporhamphus quoyi,16.28%)、叉尾鹤  (Tylosurus acus,11.99%)[30],鳙(Aristichthys nobilis,30.87%)、雷氏鲚(Coilia reynaldi,32.15%) [31];而与杜氏棱鳀(Thryssa dussumieri,35.66%)[32]、新吉富罗非鱼(NEW GIFT Oreochromis niloticus,34.51%)[33]接近。研究表明,适当增加单不饱和脂肪酸摄入量对高血压人群降低血脂水平、控制血压有益[29]。在单不饱和脂肪酸中,鸭绿江上游斑鳜肌肉中的油酸含量较高,而鸭绿江下游斑鳜肌肉中的棕榈油酸含量较高。油酸可以调节血脂水平的作用,帮助降低血清中的胆固醇含量,降低血液黏稠度[34];而棕榈油酸会影响肌肉组织的胰岛素抵抗水平,可以调节身体代谢、消炎和抑制人体细胞中的黑色素因子,改善皮肤色素沉着等[35]。鸭绿江上游和下游斑鳜肌肉中的单不饱和脂肪酸主要由油酸和棕榈油酸组成,因此高血脂和患心脑血管疾病的人群可以适当食用斑鳜,以满足自身的营养需求。
(Tylosurus acus,11.99%)[30],鳙(Aristichthys nobilis,30.87%)、雷氏鲚(Coilia reynaldi,32.15%) [31];而与杜氏棱鳀(Thryssa dussumieri,35.66%)[32]、新吉富罗非鱼(NEW GIFT Oreochromis niloticus,34.51%)[33]接近。研究表明,适当增加单不饱和脂肪酸摄入量对高血压人群降低血脂水平、控制血压有益[29]。在单不饱和脂肪酸中,鸭绿江上游斑鳜肌肉中的油酸含量较高,而鸭绿江下游斑鳜肌肉中的棕榈油酸含量较高。油酸可以调节血脂水平的作用,帮助降低血清中的胆固醇含量,降低血液黏稠度[34];而棕榈油酸会影响肌肉组织的胰岛素抵抗水平,可以调节身体代谢、消炎和抑制人体细胞中的黑色素因子,改善皮肤色素沉着等[35]。鸭绿江上游和下游斑鳜肌肉中的单不饱和脂肪酸主要由油酸和棕榈油酸组成,因此高血脂和患心脑血管疾病的人群可以适当食用斑鳜,以满足自身的营养需求。
多不饱和脂肪酸是人体不能自行合成的物质,属于必需脂肪酸,因此鱼类肌肉中多不饱和脂肪酸含量被认为是衡量鱼肉品质的重要指标[36]。高含量的多不饱和脂肪酸能明显增加肉质的香味,而且在一定程度上可提高口感[37]。近年来的研究发现,多不饱和脂肪酸具有明显促进生长、抗肿瘤、降“三高”(高血脂、高血压、高血糖)和免疫调节作用,能降低心血管疾病的发病率[38-39]。本研究发现,鸭绿江斑鳜肌肉中多不饱和脂肪酸总含量(29.922%、30.181%)高于翘嘴鲌(Culter albunus,27.89%~28.04%)[28]、野生大黄鱼(26.45%)[40]、大眼鳜(26.438%)[13] 、刀鲚(Coilia nasus,16.55%~22.65%)[41]等鱼类。鸭绿江斑鳜肌肉中的必需脂肪酸包括亚油酸和α-亚麻酸等,占总脂肪含量的比例很高(11.735%、9.571%),尤其在上游斑鳜肌肉中的含量(11.735%)显著高于刀鲚(7.28%)和湖鲚(7.94%)[25]、大眼鳜(9.780%)[13]。多不饱和脂肪酸中的亚油酸能够促进动物的生长、促进性腺成熟和保证卵巢的正常发育,α-亚麻酸具有增强智力、提高记忆力、保护视力、改善睡眠等功效[42-43],这说明鸭绿江斑鳜不仅可以满足人体对必需脂肪酸的需求,而且具有很高的保健价值。研究表明,EPA和DHA具有较高的药用价值,DHA可以促进人类脑细胞的发育,EPA在调控人的行为和情绪等方面起关键作用,二者均能刺激产生具有神经保护作用的代谢物质 [44],对妊娠妇女和儿童尤为重要。虽然亚麻酸在人体内可以转化为EPA,但速度很慢且转化量少,不能满足人体的需求,因此必需从食物中直接获得EPA的补充,而鱼源脂肪酸就是最佳来源。鸭绿江上游和下游斑鳜肌肉中EPA+DHA的总量分别为11.672%和12.939%,高于养殖鲤(Cyprinus carpio,7.74%)[45]、大菱鲆(Scophthalmus maximus,10.50%)[46],与梭鲈(Sander lucioperca,11.61%)、加州鲈(Micropterussal moides,12.3%)[47]相近。
此外,鸭绿江斑鳜肌肉中未检测出反式脂肪酸,这进一步说明鸭绿江斑鳜具有较高的食用价值和药用价值,是人类理想的健康食品。
3.3 ω-3系列、ω-6系列多不饱和脂肪酸含量及比例
4 结论
1)鸭绿江上游斑鳜肌肉中粗脂肪含量显著高于下游斑鳜(P<0.05)。
2)鸭绿江上游和下游斑鳜肌肉中不饱和脂肪酸含量均高于60%,多不饱和脂肪酸含量在29.922%~30.181%之间,ω-6/ω-3比值小于或接近1,EPA和DHA含量丰富,必需脂肪酸含量较高,无反式脂肪酸,因此其可作为膳食脂肪酸营养补充食品。相较而言,鸭绿江上游斑鳜肌肉脂肪酸营养价值较下游斑鳜高。
参考文献
4种石斑鱼肌肉中营养成分分析与评价
[J].为了测定珍珠龙胆石斑鱼(E. fuscoguttatus ♀ ×E. lanceolatus ♂)、鞍带石斑鱼(E. lanceolatus)、褐点石斑鱼(E. fuscoguttatus)和青石斑鱼(E. awoara)的营养成分,对4种石斑鱼的营养价值进行系统评价。采用国家标准生化测定方法检测石斑鱼肌肉中的水分、粗蛋白质、粗脂肪、粗灰分、氨基酸、脂肪酸及矿物质。结果表明,4种石斑鱼的蛋白质含量为(15.18%±0.21%)~(17.33%±1.09%),粗脂肪含量为(0.81%±0.03%)~(7.04%±0.21%);均检出18种氨基酸,必需氨基酸占总氨基酸含量的41.43%~42.47%,均高于联合国粮油组织与世界卫生组织(FAO/WHO)的40%评分标准,鲜味氨基酸占总氨基酸含量的41.43%~42.47%,必需氨基酸指数(EAAI值)为0.93~1.13,4种石斑鱼蛋白质质量较优;矿物质元素中,常量元素K含量最高[(3 142.50±30.41)mg/100 g~(4 209±38.18)mg/100 g],微量元素Fe含量最高[(3.51±0.32)mg/100 g~(5.91±0.14)mg/100 g];脂肪酸组成和含量中,鞍带石斑鱼检出22种脂肪酸,高于其他3种石斑鱼,其中多不饱和脂肪酸含量占总脂肪酸的38.14%,4种石斑鱼含有较高的二十二碳六烯酸和二十碳五烯酸(DHA+EPA),占脂肪酸总量的13.58%~27.57%。因此,4种石斑鱼的蛋白质质量较优,氨基酸和脂肪酸构成比例适宜,矿物质含量较为丰富,是营养价值较高的优良水产品,具有较高的综合利用价值。
闽江马口鱼肌肉营养成分分析与评价
[J].采用生化分析方法对闽江马口鱼(Opsariichthys bidens)肌肉常规营养成分和氨基酸(AA)组成与含量进行测定和分析,为闽江马口鱼的资源开发利用提供参考。结果表明:在闽江马口鱼肌肉的常规营养成分中,其水分、粗蛋白、粗脂肪和粗灰分含量依次为(75.20±0.14)%、(22.20±0.85)%、(3.15±0.02)%和(1.32±0.01)%;肌肉中检测出17种氨基酸,其中7种人体必需氨基酸(EAA)占氨基酸总量(TAA)的35.92%,鲜味氨基酸(UAA)占TAA的35.46%;EAA的氨基酸评分值(AAS)除了(Met+Cys)外均大于1,化学评分值(CS)除(Met+Cys)外均大于0.8,第一限制性氨基酸为(Met+Cys),必需氨基酸指数(EAAI)达到81.37;肌肉中微量元素含量从高到低依次为钾>磷>钙>钠>镁>锌,其中钙磷比为1.00∶4.49。综上,闽江马口鱼的营养价值较高,是一种具有良好食用价值和保健作用的优质经济鱼类。
不同生境来源的草鱼肌肉营养品质比较
[J].为了解不同生境来源草鱼肉营养品质的差异,选取了来源于河流、湖泊、高密度池塘养殖与低密度水库养殖4种不同生境草鱼,对其肌肉营养成分含量、颜色、质构特性、氨基酸、脂肪酸的组成与含量进行测定和比较分析。结果显示,2种养殖生境来源的草鱼肌肉脂肪含量显著高于2种自然生境来源的草鱼(P<0.05);肌肉硬度显著低于2种自然生境来源的草鱼(P<0.05);肌肉色差值a<sup>*</sup>值显著低于自然生境(P<0.05),b<sup>*</sup>值显著高于自然生境(P<0.05)。4种生境来源的草鱼肌肉中均以谷氨酸含量最高,鲜味氨基酸总量没有显著差异(P>0.05),高密度养殖生境草鱼肌肉氨基酸总量、必需氨基酸总量和非必需氨基酸总量较高;2种养殖生境来源的草鱼氨基酸评价较好,必需氨基酸指数值分别为96.61和90.13,高于2种自然生境来源的草鱼。4种生境草鱼肌肉均检测出18种脂肪酸,各种脂肪酸含量差异显著(P<0.05)。该研究表明,不同生境来源草鱼肌肉的营养品质差异显著,2种养殖生境草鱼氨基酸组成比自然生境的更合理,草鱼养殖过程中可以适当补充苏氨酸、甲硫氨酸和半胱氨酸,以满足草鱼生长发育的需求。
Processing technology of flavor dried flossy fish-meat
[J].
The development of muscle fiber type identity in zebrafish cranial muscles
[J].Cranial skeletal muscles underlie breathing, eating, and eye movements. In most animals, at least two types of muscle fibers underlie these critical functions: fast and slow muscle fibers. We describe here the anatomical distribution of slow and fast twitch muscle in the zebrafish (Danio rerio) head in the adult and at an early larval stage just after feeding has commenced. We found that all but one of the cranial muscles examined contain both slow and fast muscle fibers, but the relative proportion of slow muscle in each varies considerably. As in the trunk, slow muscle fibers are found only in an anatomically restricted zone of each muscle, usually on the periphery. The relative proportion of slow and fast muscle in each cranial muscle changes markedly with development, with a pronounced decrease in the proportion of slow muscle with ontogeny. We discuss our results in relation to the functional roles of each muscle in larval and adult life and compare findings among a variety of vertebrates.
Consequences of detritus type in an aquatic microsystem: effects on water quality, micro-organisms and performance of the dominant consumer
[J].1. Variation in detritus quality and quantity can have significant effects on aquatic invertebrate food webs. Allochthonous inputs of detritus are the principal energy source for organisms in aquatic tree hole microsystems. We compared the effects of two major detritus types found in tree holes, senescent leaves (Sugar Maple and White Oak) and invertebrate carcasses (dead adult fruit flies and crickets), on several water quality characteristics of laboratory microcosms as well as on mass, survival and population performance of the dominant tree hole consumer, Ochlerotatus triseriatus (Diptera: Culicidae). To date, no study has documented the effects of animal detritus in tree hole microsystems or on resident consumers.2. Aquatic environments receiving invertebrate carcasses had significantly greater total nitrogen, total reactive phosphorus and higher pH, than leaf-based environments. Decay rate of invertebrate carcasses was greater compared to leaf material. Consumption of O(2) by micro-organisms increased with increasing detritus amounts, but we detected no difference between detritus types.3. Ochlerotatus triseriatus larvae grew faster in animal-based treatments, and mean mass of larvae was significantly greater when more animal detritus was used. The effect of animal-based treatments on larvae translated into higher performance for adults, which were three times heavier than counterparts from plant-based containers. Survivorship and estimated population growth rates were significantly greater for O. triseriatus reared on animal-based versus plant-based detritus.4. We hypothesise two mechanisms for the pronounced effect of invertebrate carcasses on mosquito performance relative to that associated with leaf detritus: (i) invertebrate carcasses decompose more quickly and release nutrients more effectively into the aquatic environment; or (ii) O. triseriatus larvae may directly ingest nutrient-rich components of invertebrate carcasses. Because even relatively small animal detritus additions can have strong effects on O. triseriatus populations, studies need to be conducted to explore the overall role of animal detritus in tree holes in nature.
Lack of effect of supplementation with EPA or DHA on platelet-monocyte aggregates and vascular function in healthy men
[J].
Analysis of fatty acids in the muscle of 8 species of needlefish
[J].
Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects
[J].Elevations of postprandial triacylglycerol-rich plasma lipoproteins and suppressions of HDL-cholesterol concentrations are considered potentially atherogenic. Long-term studies have shown beneficial effects of monounsaturated fatty acids (eg, oleic acid) on fasting lipid and lipoprotein concentrations in humans. A direct stimulatory effect of oleic acid on the secretion of glucagon-like peptide 1 (GLP-1) was shown in animal studies.We compared the postprandial responses of glucose, insulin, fatty acids, triacylglycerol, gastric inhibitory polypeptide (GIP), and GLP-1 to test meals rich in saturated and monounsaturated fatty acids.Ten young, lean, healthy persons ingested 3 meals: an energy-free soup consumed with 50 g carbohydrate (control meal), the control meal plus 100 g butter, and the control meal plus 80 g olive oil. Triacylglycerol and retinyl palmitate responses were measured in total plasma, in a chylomicron-rich fraction, and in a chylomicron-poor fraction.No significant differences in glucose, insulin, or fatty acid responses to the 2 fat-rich meals were seen. Plasma triacylglycerol responses were highest after the butter meal, with chylomicron triacylglycerol rising 2.5-5-fold. Retinyl palmitate responses were higher and more prolonged after the butter meal than after the control and olive oil meals, whereas both postprandial HDL-cholesterol concentrations and GLP-1 and GIP responses were higher after the olive oil meal than after the butter meal.Olive oil induced lower triacylglycerol concentrations and higher HDL-cholesterol concentrations than butter, without eliciting differences in concentrations of glucose, insulin, or fatty acids. Furthermore, olive oil induced higher concentrations of GLP-1 and GIP than did butter, which may point to a relation between fatty acid composition, incretin responses, and triacylglycerol metabolism in the postprandial phase.
n-3 Omega fatty acids: a review of current knowledge
[J].
The effect of dietary arachidonic acid (ARA) on growth performance, fatty acid composition and expression of ARA metabolism-related genes in larval half-smooth tongue sole (Cynoglossus semilaevis)
[J].The present study was conducted to investigate the effects of dietary arachidonic acid (ARA) on growth performance, fatty acid composition and ARA metabolism-related gene expression in larval half-smooth tongue sole (Cynoglossus semilaevis). Larvae (35 d after hatching, 54 (sem1) mg) were fed diets with graded concentrations of ARA (0·01, 0·39, 0·70, 1·07, 1·42 and 2·86 % dry weight) five times per d to apparent satiation for 30 d. Results showed that increased dietary ARA concentration caused a significant non-linear rise to a plateau in survival rate, final body weight and thermal growth coefficient, and the maximum values occurred with the 1·42 % ARA treatment. As dietary ARA increased to 1·07 or 1·42 %, activities of trypsin, leucine aminopeptidase and alkaline phosphatase levels increased, but they decreased with higher ARA concentrations. The fatty acid composition of tongue sole larvae was almost well correlated with their dietary fatty acid profiles, and the EPA content of the larvae decreased with increasing dietary ARA. Meanwhile, the partial sequences ofCOX-1a(cyclo-oxygenase-1a),COX-1b(cyclo-oxygenase-1b),COX-2(cyclo-oxygenase-2),5-LOX(5-lipoxygenase) andCYP2J6-like(cytochrome P450 2J6-like) were also obtained. BothCOX-2and5-LOXmRNA expression levels significantly increased to a plateau in an ‘L’-shaped manner as dietary ARA increased to 1·07 or 1·42 %, but no significant differences were found in the gene expression ofCOX-1a,COX-1borCYP2J6-like. These results suggest that 1·07–1·42 % dietary ARA was beneficial to the growth performance of larval tongue sole, and the regulation of dietary ARA on the growth performance of larvae was probably involved in altering the mRNA expression ofCOX-2and5-LOX.
Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA,DPA and DHA
[J].
Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk
[J].The tissue composition of polyunsaturated fatty acids is important to health and depends on both dietary intake and metabolism controlled by genetic polymorphisms that should be taken into consideration in the determination of nutritional requirements. Therefore at the same dietary intake of linoleic acid (LA) and alpha-linolenic acid (ALA), their respective health effects may differ due to genetic differences in metabolism. Delta-5 and delta-6 desaturases, FADS1 and FADS2, respectively, influence the serum, plasma and membrane phospholipid levels of LA, ALA and long-chain polyunsaturated fatty acids during pregnancy, lactation, and may influence an infant's IQ, atopy and coronary heart disease (CHD) risk. At low intakes of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), polymorphisms at the 5-lipoxygenase (5-LO) level increase the risk for CHD whereas polymorphisms at cyclooxgenase-2 increase the risk for prostate cancer. At high intakes of LA the risk for breast cancer increases. EPA and DHA influence gene expression. In future, intervention studies on the biological effects of LA, ALA and LC-PUFAs, and the effects of genetic variants in FADS1 and FADS2, 5-LO and cyclooxygenase-2 should be taken into consideration both in the determination of nutritional requirements and chronic disease risk. Furthermore, genome-wide association studies need to include environmental exposures and include diet in the interaction between genetic variation and disease association.
The fats of life: essential fatty acids in health and disease
[M].
Fatty acids from marine lipids: biological activity, formulation and stability
[J].