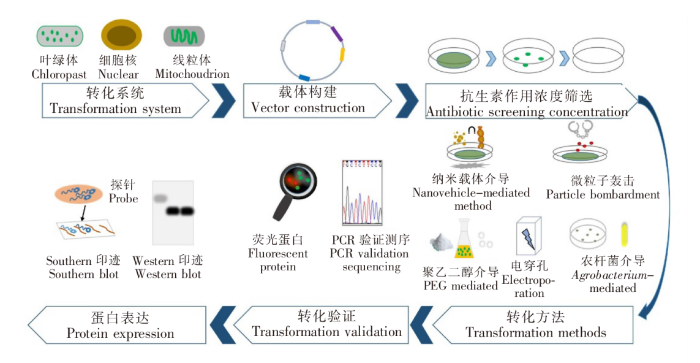
图1
作为外源基因或蛋白表达的潜在宿主,小球藻具有良好的发展前景和优势。但由于缺乏可靠高效的转化体系,蛋白表达不稳定以及高价值化合物产量相对较低,阻碍了其作为生物反应器应用的发展。因此,了解小球藻基因工程转化体系的建立和发展,对今后的真核基因表达具有积极意义。本文阐述了近年来在真核微藻小球藻中的基因工程改造进展,详细介绍了小球藻遗传转化体系的转化系统、转化方法、影响转化的因素及相关蛋白的表达,以期为后续小球藻的基因工程改造和功能蛋白生产提供技术参考。
1 小球藻转化系统
微藻中小球藻已实现细胞核和叶绿体系统的转化开发,但对于线粒体系统的转化,仅在莱茵衣藻(Chlamydomonas reinhardtii)中实现。开发转化系统的难点在于需要同时克服四个问题:1)DNA能够被转移到胞内;2)至少需要表达一个标记或报告基因;3)宿主细胞内能够复制DNA;4)被转化的细胞能够恢复和增殖。除了核转化外,小球藻叶绿体转化也逐渐受到关注。针对不同的靶细胞器转化系统,需要制定合适的转化体系,包括转化方法、启动子、标记/报告基因的选择。
1.1 核转化系统
对于小球藻的遗传转化系统,核基因组转化是最早且被应用最广泛的方法。目前已有超过12种不同的小球藻藻种成功地进行了核转化[7]。由于在细胞核中表达的基因能够编码多种功能蛋白,与核基因组严格相关的功能不能通过细胞器基因工程来修改,因此细胞核成为许多基因工程研究的主要目标[8]。对于小球藻而言,相对于细胞器转化,目前人们对核基因组的转化更为了解,操作也更为方便。Kim J等[9]利用CRISPR-Cas9系统成功编辑了小球藻的核基因组,生成硝酸还原酶(Nitrate reductase,NR)和腺嘌呤磷酸核糖转移酶(Adenine phosphoribosyl transferase,APT)基因突变体。Gomma A E等[10]利用SV40大T抗原转化功能域,通过overlap PCR技术制备线性基因表达盒,实现了该抗原成分在小球藻细胞中的稳定表达,建立了一个无抗生素标记核转化系统。Run C等[11]构建含有增强型绿色荧光蛋白(eGFP)基因的载体,通过优化电穿孔转化的相关参数,在DNA水平上成功鉴定了NptⅡ和eGFP基因,实现了蛋白核小球藻的稳定核转化。虽然小球藻的核基因组转化表达系统已逐渐成熟,但其也存在易出现基因失活、基因沉默、表达效率低等情况[12]。
1.2 叶绿体转化系统
针对植物细胞而言,其遗传物质不仅存在于细胞核中,还存在于叶绿体和线粒体中。1988年Boynton J E等[13]使用轰击法成功实现了莱茵衣藻的叶绿体转化,为随后小球藻的叶绿体转化提供了可能。Wang K等[6]通过基因枪法将两个抗菌肽基因传递到普通小球藻(C.vulgaris)的叶绿体基因组中,成功构建了小球藻叶绿体转化体系。在叶绿体转化系统中,外源DNA两侧通常有与整合位点两端同源的区域,通过同源重组将外源基因整合到叶绿体基因组的特定位点上。需要注意的是,这些同源区域要足够短,以尽量减少自发重组。叶绿体转化系统具有基因拷贝数多、表达可靠、无基因沉默现象等优点,但外源DNA的进入需要穿过叶绿体膜等障碍,这可能是小球藻叶绿体转化体系还不够成熟的原因之一。
1.3 线粒体转化系统
2 转化方法
目前,已有关于小球藻的转化方法的研究,但选择合适的转化方法仍然是实现外源蛋白在小球藻中表达的关键步骤之一。1991年,Jarvis E E等[17]使用聚乙二醇(PEG)介导法在椭圆小球藻(C. ellipsoidea)中实现了荧光素酶(Luciferase)基因的瞬时表达,这是在小球藻中首次实现核转化。之后,对于小球藻转化方法的研究逐渐增多。通过开发并优化一种可靠高效的转化方法,可以大大提高小球藻的转化效率。本文总结了5种转化方法,分析并阐述其优缺点和应用。
2.1 聚乙二醇介导法
聚乙二醇(Polyethylene glycol mediated method,PEG)介导法,又被称为玻璃珠法,是通过利用聚乙二醇与玻璃珠协同作用,造成细胞膜产生瞬间空隙,从而使外源 DNA 得以进入细胞的方法[18]。PEG介导法使用设备简单、操作方便,对宿主细胞的损伤较小[7],是最早用于使外源基因转移到微藻的方法之一,最初被用于模式藻莱茵衣藻的转化[19],之后在一些小球藻种的转化体系中也得到了应用。Hawkins R L等[20]利用PEG介导法,将带有胞外分泌信号序列和外源DNA的质粒转化到小球藻细胞中,在小球藻中实现了人生长激素(Human growth hormone,HGH)基因的瞬时表达,并发现玻璃珠的大小也会影响转化效率。杨博[18]利用PEG介导法,首次将增强型绿色荧光蛋白报告基因转化到普通小球藻基因组中,最后获得(356±30)个克隆子/μg DNA,建立了一套稳定而可靠的小球藻外源基因转化体系。然而,PEG介导法的转化效率通常较低[21],且只能用于无细胞壁细胞。因此,在使用该方法时,需要选用无细胞壁突变体作为受体细胞,或使用物理、化学、生物等方法[22]去除细胞壁以获得原生质体。目前,多数研究采用复合酶解液或物理方法(如研磨或超声破碎)对小球藻的细胞壁进行破坏和去除。
2.2 电穿孔法
电穿孔是一种常用的细胞遗传转化方法,通过施加特定强度的脉冲电场,改变细胞膜的表面结构,在细胞膜上打开微孔,以便外源 DNA 通过此孔进入受体细胞,并在恢复状态后整合到细胞染色体组中[23]。电穿孔法是目前小球藻遗传转化研究中应用最为广泛的方法,具有操作简便、转化效率较高等优点。
电穿孔法的转化效率受到多种因素的影响,包括细胞壁、电场强度、脉冲时间、质粒浓度等。Chow K C等[24]发现,在较高的电场强度下,获得的转化效果更好,但是电压过高会导致重组质粒无法稳定整合到细胞染色体组中。Muñoz C F等[25]在开发预测微藻最佳电穿孔条件的方法时发现:较低的细胞浓度、光强和小DNA片段的传递可以产生更高的转化效率。但有些种属,比如三角褐指藻(Phaeodactylum tricornutum)[26],由于其具有硅质化的细胞壁,因此无法使用电穿孔直接进行转化。针对这种情况,通常采用制备原生质体或使用渗透液渗透细胞壁,如使用山梨醇和甘露醇预先处理受体细胞,改变细胞壁通透性,以此增加质粒 DNA 进入细胞的概率。
2.3 微粒子轰击法
微粒子轰击法,又叫基因枪法,是一种可以将DNA递送到完整细胞的有效方法。它通过利用高压氦气或氮气瞬间产生的冲击力,向细胞中发射黏附有质粒DNA的金属(钨或金)微粒子,从而穿透细胞壁进入细胞[27]。Chen Y[28]等最初在椭圆小球藻中利用微粒子轰击实现了β-葡萄糖苷酸酶(β-Glucuronidase,GUS)基因的瞬时表达,表明微粒子轰击是一种用于小球藻转化的可行方法。Wang K等[6]通过直径约550 nm的金载体微颗粒将重组质粒轰击转化至普通小球藻的叶绿体中,获得了(212±48)个克隆子/μg DNA的转化值。赵熙宁[29]通过微粒子轰击介导蛋白核小球藻(C.pyrenoidosa)的转化,最终获得了高蛋白产量的优质蛋白核小球藻工程株。
微粒子轰击法的影响因素主要包括微粒子直径、轰击距离和DNA浓度等。虽然需要使用昂贵的基因枪设备,但操作简单、适用范围广。叶绿体基因组转化的唯一有效方法是微粒子轰击或生物法[19]。但使用的金属颗粒一般带有生物毒性,可能会对受体细胞产生损伤。
2.4 农杆菌介导法
农杆菌介导法是一种常用于植物遗传转化的方法,其原理是将目的基因插入到根瘤农杆菌(A.tumefaciens)Ti质粒上的T-DNA 区,通过农杆菌侵染将目的基因导入到植物细胞的核基因组中[30]。近些年来,研究发现该方法也可用于小球藻的遗传转化。Sharma P K等[31]首次报道了一种在索罗金小球藻(C.sorokiniana)中高效稳定的农杆菌介导转化体系,最终获得了(220±5)个克隆子/106细胞的转化值。冯兴标等[32]通过农杆菌介导法有效提高了小球藻中虾青素的含量。与其他方法相比,农杆菌介导法具有操作简便、宿主范围广泛、可转移大片段DNA,以及准确将低拷贝数转基因整合到转录活性区域等显著优势[33]。
2.5 纳米载体介导法
3 转化影响因素
3.1 细胞壁
若使用农杆菌或PEG介导法进行转化,需要获得细胞壁缺陷型藻株或制备原生质体。酶解法是制备藻细胞原生质体的常用方法,具有条件温和、对细胞损伤程度小等优点。纤维素是大多数小球藻种细胞壁的主要成分[39]。研究表明,同时使用纤维素酶和崩溃酶预处理小球藻,可以显著提高原生质体制备率[39]。Yang B等[5]使用了含有纤维素酶、离析酶和果胶酶的酶混合液预处理小球藻的细胞壁,最终得到了超过80%的原生质体产率。尽管破坏细胞壁不是所有藻类成功转化的必要条件,但它是细胞渗透到足以有效穿透外源DNA和细胞死亡之间的微妙平衡[8],可以有效提高转化效率。在莱茵衣藻中,高效率转化通常只报道在无细胞壁突变体或已去除细胞壁的原生质体细胞中[8]。因此,在小球藻基因转化研究中,细胞壁的处理是提高转化效率的关键步骤之一。
3.2 选择标记和报告基因
在转化体系中,通常会将带有某一特定抗性的选择标记或带有荧光报告基因的质粒与目的DNA融合后,转化到细胞中,便于后续阳性转化子的筛选。选择标记是指将编码某一抗生素的抗性基因导入细胞中表达,从而使转化细胞具有对该抗生素的抗性特质。表1列出了目前常用于小球藻遗传转化筛选的抗生素抗性选择标记。
表1 用于不同种类小球藻的抗生素抗性基因及最佳筛选浓度
Tab.1
| 小球藻藻种 Chlorella species | 抗性基因 Resistant genes | 抗生素 Antibiotic | 最佳筛选浓度/mg·mL-1 Optimal screening concentration | 参考文献 Reference |
|---|---|---|---|---|
| 普通小球藻C.vulgaris | aadA | 奇霉素 | 0.10~0.20 | [6] |
| 椭圆小球藻C.ellipsoidea | NptⅡ | G418 | 0.03 | [36] |
| 蛋白核小球藻C.pyrenoidosa | aadA | 硫酸链霉素 | 0.10 | [27] |
| 蛋白核小球藻C.pyrenoidosa | 卡那霉素 | 1.00 | [29] | |
| 小球藻Chlorella | Kanr | G418 | 1.00 | [20] |
| 椭圆小球藻C.ellipsoidea | Sh ble | 腐草霉素 | 0.001 | [40] |
| 普通小球藻C.vulgaris | hpt | 潮霉素 | 0.02 | [41] |
| 小球藻Chlorella sp. | hpt | 潮霉素B | 0.05 | [21] |
| 普通小球藻C.vulgaris | hpt | 潮霉素B | 0.30 | [42] |
| 蛋白核小球藻C.pyrenoidosa | NptⅡ | G418 | 0.03 | [11] |
| 普通小球藻C.vulgaris | NptⅡ | G418 | 0.03 | [5] |
| 椭圆小球藻C.ellipsoidea | NptⅡ、NR | G418 | 0.03 | [43] |
| 椭圆小球藻C.ellipsoidea | ble | 博来霉素 | 0.01 | [44] |
| 普通小球藻C.vulgaris | CAT | 氯霉素 | 0.20 | [45] |
| 普通小球藻C.vulgaris | hpt | 潮霉素B | 0.02 | [33] |
| 小球藻Chlorella | neo | G418 | 0.16 | [23] |
不同的藻种对抗生素有不同的敏感浓度,在选择抗生素时,需要提前考虑藻的种类和其本身的抗性,并设置一系列抗生素浓度梯度,观察抑制野生型藻株生长的最佳作用浓度,这是小球藻基因工程改造中的重要部分。
除了肉眼可以观察到的显性选择标记外,还有一些隐性选择标记可用于筛选转化子。NR基因编码了一种介导硝酸盐向亚硝酸盐转化的酶,NR基因突变体不能利用硝酸盐,因此在仅以硝酸盐为氮源的培养基中无法生长。另外,APT基因编码一种催化腺嘌呤转化为腺苷酸的蛋白质,APT突变体会导致细胞在含有2-氟腺嘌呤的培养基中不受抑制地生长[9]。这种通过基因突变导致翻译提前终止,蛋白无法表达的隐性选择标记可以减少外源基因的插入和抗生素的使用,具有提高经济效益和减少环境污染等优点。
3.3 启动子和增强子
启动子是基因表达的关键因子,强启动子会调控基因的高表达[47]。花椰菜花叶病毒35S (CaMV35S)启动子是小球藻中应用最广泛的组成型启动子之一[7],已被成功用于普通小球藻[5]、椭圆小球藻[17]、蛋白核小球藻[48]。来自植物的组成型启动子也可以发挥作用。如玉米的泛素启动子已成功驱动GUS报告基因在小球藻中的高效表达[44],而水稻肌动蛋白的Actin1启动子也被认为可以促进小球藻中的基因表达。Niu Y F等[45]克隆了NR基因的启动子和终止子,驱动诱导氯霉素乙酰转移酶(Chloramphenicol acetyltransferase,CAT)报告基因的表达,结果显示CAT的表达会受到硝酸盐的诱导,提出了一种诱导型启动子在小球藻中的应用体系。
一般来说,外源启动子的表达效率低于内源启动子。在小球藻中被鉴定并分离出的光系统Ⅰ蛋白D (psaD)启动子,是首次报道的可以驱动真核微藻和高等植物基因表达[49]的小球藻内源性启动子。Shin J H等[50]从小球藻核基因组中分离到两个氮饥饿诱导型启动子,通过添加信号肽,成功实现了人粒细胞集落刺激因子(hG-CSF)多肽的表达和分泌。为了高效驱动基因表达,研究者会在构建质粒时加入一些调控元件,如增强子来激活启动子发挥功能。研究表明在中性粒细胞肽(Neutrophil peptide-1,NP-1)基因上游区域添加一个
4 蛋白表达
小球藻拥有完整的真核细胞表达系统,因此可以对外源蛋白进行翻译后修饰和加工。此外,小球藻具有生长周期短、食用安全性高、生产效率高等优势,被广泛应用于基因工程领域。表2列出了不同种类小球藻转化系统中的转化率或蛋白表达量。
表2 不同种类小球藻的转化率或蛋白表达量
Tab.2
| 小球藻藻种 Chlorella species | 表达载体 Vector | 启动子 Promoter | 转化方法 Transformation method | 转化率或蛋白表达量 Transformation rate or protein expression | 参考文献 Reference |
|---|---|---|---|---|---|
| 普通小球藻 C.vulgaris | pUC改造的 pCMCC | Prrn | 电穿孔 | 人碱性成纤维细胞生长因子表达量0.26~1.42 ng/g藻细胞鲜重 | [37] |
| 普通小球藻 C.vulgaris | pMD18T | P16S、rbcL | 微粒子轰击 | 转化率(212±48)转化子/μg质粒DNA | [6] |
| 索罗金小球藻 C.sorokiniana | pCAMBIA1301 | CaMV35S | 农杆菌介导 | 转化率(220±5)转化子/106细胞 | [31] |
| 普通小球藻 C.vulgaris | pBYR2e | CaMV35S | 农杆菌介导 | 表达量1.14 μg/g鲜重、成纤维细胞生长因子表达量1.61 ng/g鲜重 | [53] |
| 椭圆小球藻 C.ellipsoidea | pGREEN0029、 pBAC818 | e35s | 纳米载体介导 | 转化率(0.95×102±0.07)转化子/μg质粒DNA | [36] |
| 佐夫色绿藻 C.zofingiensis | pBI121 | CaMV35S | 农杆菌介导 | 虾青素质量比 0.564 mg/g | [32] |
| 蛋白核小球藻 C.pyrenoidosa | pBI121 | 微粒子轰击 | 目的蛋白含量高达55.94% | [29] | |
| 小球藻 Chlorella sp. | pMDC85 | 2×CaMV35S | 电穿孔 | 可溶性总蛋白中目的蛋白含量约占0.052%~0.078% | [54] |
| 蛋白核小球藻 C.pyrenoidosa | pGREENII0029 | Ubi | 电穿孔 | 转化率(101±7)转化子/μg质粒DNA | [11] |
| 普通小球藻 C.vulgaris | pBI121 | CaMV35S | PEG介导 | 转化率(356±30)转化子/μg质粒DNA | [5] |
| 椭圆小球藻 C.ellipsoidea | pGREEN0029 | Ubi | 电穿孔 | 转化率750转化子/μg质粒DNA,蛋白表达量11.42 mg/L | [43] |
| 椭圆小球藻 C.ellipsoidea | pSP124 | Ubi-1、rbcS2 | 纤维素酶处理 | 转化率2.25×103转化子/μg质粒DNA | [44] |
Hawkins R L等[20]发现在小球藻中表达的异源蛋白HGH的产量约为200~600 ng/mL。虽然比报道的大肠杆菌(Escherichia coli)中的HGH(0.5~2.4 μg/mL)产量低。但该研究为在小球藻中表达生产外源蛋白提供了良好的开端。
小球藻中表达的重组蛋白可用于生产天然饵料。Kim D H等[40]在椭圆小球藻中稳定转化了比目鱼生长激素(Flounder growth hormone,fGH)基因,最后测定fGH的蛋白量超过了400 μg。将转化后的小球藻喂养比目鱼30 d后,其生长速度提高了25%。Reddy P H等[48]将含有传染性法氏囊病病毒(Infectious bursal disease virus,IBDV)VP2基因的表达载体转化到蛋白核小球藻中,Western blot分析证实了IBDV VP2蛋白可以在蛋白核小球藻中表达,该蛋白可以保护鸡免受鸡传染病病毒IBDV的侵害。这些研究证实了小球藻中外源蛋白稳定表达的可行性,为工业化生产水产养殖业的食用蛋白提供了可靠依据。
5 总结与展望
小球藻是高值生物产物和功能蛋白质的一种重要来源。通过利用各种技术工具对小球藻进行基因组编辑和蛋白表达,扩展其作为表达宿主,具有经济性和环境可行性。目前,电穿孔和基因枪法是小球藻转化的常用方法,其中基因枪法是用于叶绿体基因组转化的最有效方法。常用于植物转化系统的农杆菌介导法,不需要提前制备原生质体,在小球藻转化体系中也有巨大的应用潜力。启动子、功能表达载体和合适的选择标记是小球藻转化体系的重要组成部分。构建和利用植物病毒载体,密码子优化、插入调控元件、使用内源性启动子等操作可以提高外源基因插入的效率和稳定性,并增加蛋白表达量。
小球藻作为一种生命活动简单的真核微藻,在功能营养饵料和改善生态环境方面都有可观的前景。尽管不断的探索和研究使小球藻基因工程改造领域取得了相当大的进展,但在其成为成熟的表达系统之前仍有许多障碍需要克服。开发一种适合小球藻高效、稳定表达蛋白的转化体系,对于生产高价值产品和功能性营养品具有重要而深远的意义。
参考文献
Microalgal biofuel production: potential challenges and prospective research
[J].
Agriculture of microalgae Chlorella vulgaris for polyunsaturated fatty acids (PUFAs) production employing palm oil mill effluents (POME) for future food, wastewater, and energy nexus
[J].
Comprehensive utilization of marine microalgae for enhanced co-production of multiple compounds
[J].Marine microalgae are regarded as potential feedstock because of their multiple valuable compounds, including lipids, pigments, carbohydrates, and proteins. Some of these compounds exhibit attractive bioactivities, such as carotenoids, ω-3 polyunsaturated fatty acids, polysaccharides, and peptides. However, the production cost of bioactive compounds is quite high, due to the low contents in marine microalgae. Comprehensive utilization of marine microalgae for multiple compounds production instead of the sole product can be an efficient way to increase the economic feasibility of bioactive compounds production and improve the production efficiency. This paper discusses the metabolic network of marine microalgal compounds, and indicates their interaction in biosynthesis pathways. Furthermore, potential applications of co-production of multiple compounds under various cultivation conditions by shifting metabolic flux are discussed, and cultivation strategies based on environmental and/or nutrient conditions are proposed to improve the co-production. Moreover, biorefinery techniques for the integral use of microalgal biomass are summarized. These techniques include the co-extraction of multiple bioactive compounds from marine microalgae by conventional methods, super/subcritical fluids, and ionic liquids, as well as direct utilization and biochemical or thermochemical conversion of microalgal residues. Overall, this review sheds light on the potential of the comprehensive utilization of marine microalgae for improving bioeconomy in practical industrial application.
Valuable bioproducts obtained from microalgal biomass and their commercial applications: a review
[J].
Development of a stable genetic system for Chlorella vulgaris—a promising green alga for CO2 biomitigation
[J].
The chloroplast genetic engineering of a unicellular green alga Chlorella vulgaris with two foreign peptides co-expression
[J].
Chlorella species as hosts for genetic engineering and expression of heterologous proteins: progress, challenge and perspective
[J].The species of Chlorella represent a highly specialized group of green microalgae that can produce high levels of protein. Many Chlorella strains can grow rapidly and achieve high cell density under controlled conditions and are thus considered to be promising protein sources. Many advances in the genetic engineering of Chlorella have occurred in recent years, with significant developments in successful expression of heterologous proteins for various applications. Nevertheless, a lot of obstacles remain to be addressed, and a sophisticated and stable Chlorella expression system has yet to emerge. This review provides a brief summary of current knowledge on Chlorella and an overview of recent progress in the genetic engineering of Chlorella, and highlights the advances in the development of a genetic toolbox of Chlorella for heterologous protein expression. Research directions to further exploit the Chlorella expression system with respect to both challenges and perspectives are also discussed. This paper serves as a comprehensive literature review for the Chlorella community and will provide valuable insights into future exploration of Chlorella as a promising host for heterologous protein expression.Copyright © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Methodological review of genetic engineering approaches for non-model algae
[J].
Establishment of a genome editing tool using CRISPR-Cas9 in Chlorella vulgaris UTEX395
[J].
Development of stable marker-free nuclear transformation strategy in the green microalga Chlorella vulgaris
[J].
Stable nuclear transformation of the industrial alga Chlorella pyrenoidosa
[J].
Chloroplast transformation in Chlamydomonas with high velocity microprojectiles
[J].Bombardment of three mutants of the chloroplast atpB gene of Chlamydomonas reinhardtii with high-velocity tungsten microprojectiles that were coated with cloned chloroplast DNA carrying the wild-type gene permanently restored the photosynthetic capacity of the algae. In most transformants of one of the mutants, a fragment with a 2.5-kilobase deletion was restored to normal size by a homologous replacement event; in about 25 percent of the transformants the restored restriction fragment was 50 to 100 base pairs smaller or larger than that of wild type. About one-fourth of the transformants of this mutant contained unintegrated donor plasmid when first examined. This plasmid persisted in four different transformants after 65 cell generations of continuous liquid culture but was lost from all transformants maintained on plates of selective medium. The restored wild-type atpB gene remains in all transformants as an integral part of the chloroplast genome and is expressed and inherited normally.
Transformation of the mitochondrial genome
[J].Although mitochondrial transformation is highly desirable in mammals and plants, it is only possible in two unicellular organisms, the budding yeast Saccharomyces cerevisiae and the unicellular green alga Chlamydomonas reinhardtii. Here, we give an overview of the attempts made to transform mitochondria of mammals and plants and the possible reasons for their failure. This review briefly describes the mitochondrial transformation principles in yeast and describes in more detail the transformation and its applications in Chlamydomonas.
Stable expression of antibiotic-resistant gene ble from Streptoalloteichus hindustanus in the mitochondria of Chlamydomonas reinhardtii
[J].
Cas9/gRNA-mediated genome editing of yeast mitochondria and Chlamydomonas chloroplasts
[J].We present a new approach to edit both mitochondrial and chloroplast genomes. Organelles have been considered off-limits to CRISPR due to their impermeability to most RNA and DNA. This has prevented applications of Cas9/gRNA-mediated genome editing in organelles while the tool has been widely used for engineering of nuclear DNA in a number of organisms in the last several years. To overcome the hurdle, we designed a new approach to enable organelle genome editing. The plasmids, designated “Edit Plasmids,” were constructed with two expression cassettes, one for the expression of Cas9, codon-optimized for each organelle, under promoters specific to each organelle, and the other cassette for the expression of guide RNAs under another set of promoters specific to each organelle. In addition, Edit Plasmids were designed to carry the donor DNA for integration between two double-strand break sites induced by Cas9/gRNAs. Each donor DNA was flanked by the regions homologous to both ends of the integration site that were short enough to minimize spontaneous recombination events. Furthermore, the donor DNA was so modified that it did not carry functional gRNA target sites, allowing the stability of the integrated DNA without being excised by further Cas9/gRNAs activity. Edit Plasmids were introduced into organelles through microprojectile transformation. We confirmed donor DNA insertion at the target sites facilitated by homologous recombination only in the presence of Cas9/gRNA activity in yeast mitochondria and Chlamydomonas chloroplasts. We also showed that Edit Plasmids persist and replicate in mitochondria autonomously for several dozens of generations in the presence of the wild-type genomes. Finally, we did not find insertions and/or deletions at one of the Cas9 cleavage sites in Chloroplasts, which are otherwise hallmarks of Cas9/gRNA-mediated non-homologous end joining (NHEJ) repair events in nuclear DNA. This is consistent with previous reports of the lack of NHEJ repair system in most bacteria, which are believed to be ancestors of organelles. This is the first demonstration of CRISPR-mediated genome editing in both mitochondria and chloroplasts in two distantly related organisms. The Edit Plasmid approach is expected to open the door to engineer organelle genomes of a wide range of organisms in a precise fashion.
Transient expression of firefly luciferase in protoplasts of the green alga Chlorella ellipsoidea
[J].
Advances and challenges in genetic engineering of microalgae
[J].
Expression of human growth hormone by the eukaryotic alga,Chlorella
[J].A method to use Chlorella to express a recombinant heterologous protein that can be recovered from the extracellular medium has been developed. Plasmids are constructed with an extracellular secretion signal sequence inserted between a promoter region and a gene for human growth hormone (hGH). The plasmids also contain a Kanr region which confers resistance to the antibiotic G418. Protoplasts are prepared by enzymatic treatment, and the plasmid is introduced by incubation of the protoplasts with polyethylene glycol and dimethyl sulfoxide. Cells are then grown in the presence of G418, and the medium is collected from 6 days after transfection. hGH is measured by immunoassay, and values for expressed hGH of about 200-600 ng/ml are obtained.
Expression of mercuric reductase from Bacillus megaterium MB1 in eukaryotic microalga Chlorella sp. DT: an approach for mercury phytoremediation
[J].
Microalgae disruption techniques for product recovery: influence of cell wall composition
[J].Microalgae are one of the most promising feedstocks for the production of commodity and value-added products. However, the use of microalgae as a feedstock is hampered by the process economics and sustainability. Overall sustainability can be improved by employing energy-efficient cell disruption and recovery processes to maximize the extraction of desired compounds from microalgal biomass. Most often, extraction processes are conducted with solvents using untreated, chemically treated, or mechanically treated cells. However, microalgal cell walls are sometimes structurally robust, complex, and chemically diverse and high energy inputs or large quantities of chemicals are required to extract products from within the cell. Various chemical, biological, and physical techniques have been employed to disrupt the cell walls from a variety of microalgae species, and these include the use of surfactants, autoclave, microwave, sonication, bead milling, enzymatic hydrolysis, high-pressure homogenization, and steam treatments. Although the cell wall structure is important for product recovery, it is often not considered when selecting the most appropriate disruption method. In this study, the cell wall structure of selected microalgae species and the effectiveness of various cell wall disruption techniques on product recovery are reviewed. It was concluded that future research must focus on developing an understanding of the relationship between cell wall disruption mechanisms and cell wall composition and structure, as well as optimizing the energy consumption of the disruption technique. This approach would enable the design of innovative cell wall disruption techniques for an enhanced product recovery.
Electrotransformation of Chlorella vulgaris
[J].
Improved DNA/protein delivery in microalgae—a simple and reliable method for the prediction of optimal electroporation settings
[J].
Study on transient expression of gus gene in Chlorelia ellipsoidea (Chlorophyta) by using biolistic particle delivery system
[J].
Efficient Agrobacterium tumefaciens-mediated stable genetic transformation of green microalgae, Chlorella sorokiniana
[J].
Assessment of factors affecting Agrobacterium-mediated genetic transformation of the unicellular green alga, Chlorella vulgaris
[J].
Factors affecting Agrobacterium mediated transformation of indigenous Chlorella vulgaris Bayerinck
[J].Chlorella vulgarisis Bayerinck widely used as a health food, feed supplement, as well as in the pharmaceutical, biofuel and cosmetics industries. It has been used to determine optimum transformation conditions through Agrobacterium tumefaciens. It has been revealed that bacterial density of OD600 = 1.0, 3 days of co-cultivation at 25°C in pH 5.5, and 100 μM acetosyringone are the optimum conditions to transform C. vulgaris.
Magnetic nanoparticles mediate the transformation of antimicrobial peptides HeM into Chlorella ellipsoidea
[J].
Construction and validation of a chloroplast expression vector for the production of recombinant proteins in Chlorella vulgaris
[J].
Rapid and efficient genetic transformation of the green microalga Chlorella vulgaris
[J].
Breeding of high protein Chlorella sorokiniana using protoplast fusion
[J].
Stable integration and functional expression of flounder growth hormone gene in transformed microalga, Chlorella ellipsoidea
[J].
Expression of bovine lactoferrin N-lobe by the green alga, Chlorella vulgaris
[J].
Production of crocetin in transgenic Chlorella vulgaris expressing genes crtRB and ZCD1
[J].
A new strategy to produce a defensin: stable production of mutated NP-1 in nitrate reductase-deficient Chlorella ellipsoidea
[J].
Development of a new method for genetic transformation of the green alga Chlorella ellipsoidea
[J].
A new inducible expression system in a transformed green alga, Chlorella vulgaris
[J].Genetic transformation is useful for basic research and applied biotechnology. However, genetic transformation of microalgae is usually quite difficult due to the technical limitations of existing methods. We cloned the promoter and terminator of the nitrate reductase gene from the microalga Phaeodactylum tricornutum and used them for optimization of a transformation system of the microalga Chlorella vulgaris. This species has been used for food production and is a promising candidate as a bioreactor for large-scale production of value-added proteins. A construct was made containing the CAT (chloramphenicol acetyltransferase) reporter gene driven by the nitrate reductase promoter. This construct was transferred into the C. vulgaris genome by electroporation. Expression of CAT in transgenic Chlorella conferred resistance to the antibiotic chloramphenicol and enabled growth in selective media. Overall efficiency for the transformation was estimated to be approximately 0.03%, which is relatively high compared with other available Chlorella transformation systems. Expression of CAT was induced in the presence of nitrate and inhibited in the presence of ammonium as a sole nitrogen source. This study presented an inducible recombinant gene expression system, also providing more gene regulation elements with potential for biotechnological applications.
Transient expression of the GUS gene in a unicellular marine green alga, Chlorella sp. MACC/C95, via electroporation
[J].
Advantages of heterotrophic microalgae as a host for phytochemicals production
[J].Currently, most commercial recombinant technologies rely on host systems. However, each host has their own benefits and drawbacks, depending on the target products. Prokaryote host is lack of post-transcriptional and post-translational mechanisms, making them unsuitable for eukaryotic productions like phytochemicals. Even there are other eukaryote hosts (e.g., transgenic animals, mammalian cell, and transgenic plants), but those hosts have some limitations, such as low yield, high cost, time consuming, virus contamination, and so on. Thus, flexible platforms and efficient methods that can produced phytochemicals are required. The use of heterotrophic microalgae as a host system is interesting because it possibly overcome those obstacles. This paper presents a comprehensive review of heterotrophic microalgal expression host including advantages of heterotrophic microalgae as a host, genetic engineering of microalgae, genetic transformation of microalgae, microalgal engineering for phytochemicals production, challenges of microalgal hosts, key market trends, and future view. Finally, this review might be a directions of the alternative microalgae host for high-value phytochemicals production in the next few years.
Heterologous expression of infectious bursal disease virus VP2 gene in Chlorella pyrenoidosa as a model system for molecular farming
[J].
Identification and functional analysis of the psaD promoter of Chlorella vulgaris using heterologous model strains
[J].
The establishment of new protein expression system using N starvation inducible promoters in Chlorella
[J].Chlorella is a unicellular green microalga that has been used in fields such as bioenergy production and food supplementation. In this study, two promoters of N (nitrogen) deficiency-inducible Chlorella vulgaris N Deficiency Inducible (CvNDI) genes were isolated from Chlorella vulgaris UTEX 395. These promoters were used for the production of a recombinant protein, human granulocyte-colony stimulating factor (hG-CSF) in Chlorella vulgaris UTEX 395 and Chlorella sp. ArM0029B. To efficiently secrete the hG-CSF, the protein expression vectors incorporated novel signal peptides obtained from a secretomics analysis of Chlorella spp. After a stable transformation of those vectors with a codon-optimized hG-CSF sequence, hG-CSF polypeptides were successfully produced in the spent media of the transgenic Chlorella. To our knowledge, this is the first report of recombinant protein expression using endogenous gene components of Chlorella.
Highly efficient expression of rabbit neutrophil peptide-1 gene in Chlorella ellipsoidea cells
[J].A highly efficient system was developed for the expression of foreign genes in Chlorella ellipsoidea cells. The effect of five promoters on the expression efficiency of beta-glucuronidase (GUS) gene was evaluated by transient expression of the UidA gene. Among these promoters, Ubiquitin-omega was found to be the most efficient and was selected to drive the expression of foreign genes in Chlorella cells. A gene encoding the mature rabbit neutrophil peptide-1 (NP-1) was introduced into the cells. Integration of the gene for NP-1 into the Chlorella genome was confirmed by PCR and Southern blot analysis. In, vitro anti-microbial testes demonstrated the expression of biologically active NP-1 by the transgenic Chlorella cells.
Genetic engineering of the green alga Chlorella zofingiensis: a modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker
[J].
Efficient transient expression of recombinant proteins using DNA viral vectors in freshwater microalgal species
[J].The increase in the world population, the advent of new infections and health issues, and the scarcity of natural biological products have spotlighted the importance of recombinant protein technology and its large-scale production in a cost-effective manner. Microalgae have become a significant promising platform with the potential to meet the increasing demand for recombinant proteins and other biologicals. Microalgae are safe organisms that can grow rapidly and are easily cultivated with basic nutrient requirements. Although continuous efforts have led to considerable progress in the algae genetic engineering field, there are still many hurdles to overcome before these microorganisms emerge as a mature expression system. Hence, there is a need to develop efficient expression approaches to exploit microalgae for the production of recombinant proteins at convenient yields. This study aimed to test the ability of the DNA geminiviral vector with Rep-mediated replication to transiently express recombinant proteins in the freshwater microalgal species Chlamydomonas reinhardtii and Chlorella vulgaris using Agrobacterium-mediated transformation. The SARS-CoV-2 receptor binding domain (RBD) and basic fibroblast growth factor (bFGF) are representative antigen proteins and growth factor proteins, respectively, that were subcloned in a geminiviral vector and were used for nuclear transformation to transiently express these proteins in C. reinhardtii and C. vulgaris. The results showed that the geminiviral vector allowed the expression of both recombinant proteins in both algal species, with yields at 48 h posttransformation of up to 1.14 μg/g RBD and 1.61 ng/g FGF in C. vulgaris and 1.61 μg/g RBD and 1.025 ng/g FGF in C. reinhardtii. Thus, this study provides a proof of concept for the use of DNA viral vectors for the simple, rapid, and efficient production of recombinant proteins that repress the difficulties faced in the genetic transformation of these unicellular green microalgae. This concept opens an avenue to explore and optimize green microalgae as an ideal economically valuable platform for the production of therapeutic and industrially relevant recombinant proteins in shorter time periods with significant yields.
Evaluation of microalgae as immunostimulants and recombinant vaccines for diseases prevention and control in aquaculture
[J].
Multiomics approaches and genetic engineering of metabolism for improved biorefinery and wastewater treatment in microalgae
[J].