甲苯咪唑(Mebendazole,MBZ)是一种用于治疗人和动物体内各种寄生虫的苯并咪唑类驱虫药,以及用于对抗蠕虫,如钩虫、鞭虫和蛔虫等[1],结构见图1。它通过阻断虫体肠细胞浆微管的形成和营养吸收而发挥作用。自1998年被发现具有良好的治疗平鲉单殖吸虫效果[2]后,MBZ逐步被应用于水产寄生虫的驱杀[3],实践结果也证明其具有良好的杀虫效果[2,4⇓-6]。然而,MBZ及其代谢物对人类和动物具有潜在毒性,并已被证明对哺乳动物具有致畸作用[7-8]。我国也曾报道过多例使用MBZ引起的脑病综合征病例,引起了人们对MBZ的关注[9]。鉴于MBZ的毒性作用,欧盟和国际食品法典委员会发布了相应的安全标准,用于监测动物食品中的MBZ残留;美国食品药品监督管理局禁止其在孕妇人群中及动物怀孕期间被使用。同样,我国也对动物源性食品中MBZ残留作出了相应的规定,MBZ在羊、马的肌肉和脂肪的最大残留限量(Maximum residue limit,MRL)为 60 μg/kg,在肝脏、肾脏中的MRL分别为400 μg/kg和60 μg/kg[10],这与欧盟标准一致。
图1
虽然 MBZ 已经在水产养殖中批准使用,但由于缺乏完善的相关药代动力学研究的基础数据,我国并未规定MBZ在鱼组织中的MRL。目前,国内有关MBZ的药代动力学研究不多,仅见在鲫(Carassius auratus)、欧洲鳗鲡(Anguilla anguilla)、鳗鲡(A.japonica)等鱼类,给药模式也集中在口灌和浸浴两种方式。潘浩等[11]以连续3 d单剂量口灌20 mg/kg(体质量)MBZ的给药方式,研究其在鲫体内的吸收及残留消除规律,结果表明其药时关系符合一级吸收二室开放模型,建议休药期为25 d,但该种模式并不符合实际养殖用药方式,对实际生产的指导意义不大。廖碧钗等[12]采用浸浴(1 000 μg/L)方式开展了MBZ及其代谢物在欧洲鳗鲡体内药物动力学及残留研究,结果表明MBZ在各组织中的残留量及消除速度差别较大,建议休药期不低于48 d。李忠琴等[13]报道了在水温20℃、1 000 μg/L MBZ药浴后,鳗鲡体内的药物代谢及组织残留情况,虽然该浸浴方式与实际养殖给药方式(全池泼洒)基本相似,但研究忽略了用药时间。为了保证MBZ的药效,实际养殖中MBZ的使用建议为5 d不换水。而受养殖条件的限制,某些养殖户对养殖对象的用药时间可能持续更久。
浸浴药物浓度是影响药物代谢研究的关键参数[14],因此实际养殖水体中MBZ的含量变化与鱼组织中MBZ残留量息息相关。本文结合实际养殖用药方式,进一步研究了长期暴露于MBZ下的养殖水体中MBZ的动态消除规律,以期为水产养殖动物中MBZ的药代动力学研究提供基础数据。
1 材料与方法
1.1 材料与试剂
MBZ、MBZ-d3标准品(纯度99%),德国Dr.Ehrensorfer公司;上述标准品分别用甲醇溶解定容至10 000 μg/L标准储备液,-18℃避光保存,有效期为12个月;用甲醇分别稀释标准储备液至1 000、100 μg/L MBZ标准中间液,以及100 μg/L MBZ-d3标准中间液,4℃避光保存,有效期为6个月。
MBZ原粉(纯度98%),上海安谱科学仪器有限公司;吐温80,上海阿拉丁生化科技股份有限公司;甲醇、甲酸、乙酸乙酯(均为色谱纯),美国Tidea公司;浓氨水(分析纯),国药集团化学试剂有限公司;0.22 μm尼龙微孔滤膜,天津市津腾实验设备有限公司。
定容液配制:0.1%甲酸水溶液65 mL,加入甲醇35 mL,混匀,置于4℃冰箱中备用。
1.2 仪器与设备
TSQ QUANTUMULTRA高效液相色谱-串联质谱仪(HPLC-MS/MS),美国Thermo-Fisher Scientific公司;AB204-E型、PL203型电子分析天平,美国Mettler Toledo公司; R系列旋转蒸发仪,上海申生科技有限公司;MS3涡旋振荡器,德国IKA公司;KQ3200E超声波清洗仪,昆山市超声仪器有限公司;Milli-Q型超纯水仪,美国Millipore公司。
1.3 实验条件
为了更好地模拟重现实际养殖条件,冬季在户外围隔池塘开展海水中MBZ消除实验。实验水体温度为15~18℃,实验用水为曝气 48 h的海水,连续充氧,保持水中溶解氧浓度大于5.0 mg/L,pH为6.8~7.5。实际日照天数为27 d,日照时数为6~7 h/d,光强为冬季自然日照强度。
1.4 试样采集
低浓度组(15 μg/L):称取 0.3 g MBZ 原粉,加入10 mL甲酸,置涡旋振荡器上振荡,使 MBZ 充分溶解在甲酸里,加入 20 mL 吐温 80,再加水稀释至 500 mL。使用时泼洒至盛有20 m3曝气水的室外实验池中,并连续充氧,水温为15~18℃,pH值约6.8。
高浓度组(50 μg/L):称取 1.0 g MBZ 原粉,加入25 mL甲酸,置涡旋振荡器上振荡,使 MBZ 充分溶解在甲酸里,加入 60 mL 吐温 80,再加水稀释至 500 mL。使用时泼洒至盛有20 m3曝气水的室外实验池中,并连续充氧,水温为15~18℃,调节pH值至6.8。
对照避光组(15 μg/L):称取 0.015 g MBZ 原粉,加入0.5 mL甲酸,置涡旋振荡器上振荡,使 MBZ 充分溶解在甲酸里,加入 1 mL 吐温 80,再加水稀释至 25 mL。使用时泼洒至盛有1 m3曝气水的室外实验池中,池口用铝箔封住避光,并连续充氧,水温为15~18℃。
采样:在泼洒MBZ后的 0、72、120、168、216、288、384、504、648 h,各采集1份水样。
1.5 样品前处理方法
取50 mL水样于250 mL分液漏斗中,加入100 μL 100 μg/L MBZ-d3内标溶液,混匀后再加入浓氨水调节pH至中性;再加50 mL乙酸乙酯,振荡混匀 1 min,静置分层后取上层萃取液于鸡心瓶中,重复振荡提取2次,再合并上层萃取液于鸡心瓶中;40℃下减压旋转蒸发至干。1 mL 定容液超声溶解1 min,经 0.22 μm 尼龙微孔滤膜过滤,HPLC-MS/MS 测定。
1.6 HPLC-MS/MS条件
色谱条件:Hypersil Gold-C18色谱柱(2.1 mm×150 mm×3 μm);柱温30℃;流速0.25 mL/min;进样量10 μL;流动相:A 为0.1%甲酸水溶液,B 为甲醇;洗脱梯度:0~0.2 min(35%~50% B)、0.2~3.0 min(50%~65% B)、3.0~6.6 min(65% B)、6.6~7.0 min(65%~35%B)、7.0~12.0 min(35% B)。
质谱条件:电喷雾离子源,正离子检测模式,雾化室加热温度:150℃,喷雾电压:3 500 V,鞘气压力:267 kPa,辅助气压力:5 L/min,离子传输毛细管温度:350℃,选择反应监测模式(SRM),MBZ监测离子为296→77(碰撞能量28 eV)和296→105(碰撞能量29 eV),MBZ-d3监测离子为299→105(碰撞能量32 eV),Q1半峰宽:0.7 u,Q3半峰宽:0.7 u,碰撞气压力:氩气、0.2 Pa。
1.7 质量控制
先依据保留时间和特征离子,准确地计算标准曲线,再进行样品定量分析。移取适量标准溶液,用定容液分别配制成不同质量浓度标准溶液,目标物浓度分别为0.5、2.5、5.0、25、50、100、200 μg/L,内标MBZ-d3质量浓度为50 μg/L。本方法采用内标法定量,以标准溶液质量浓度为横坐标、目标物和内标物的峰面积比值为纵坐标,绘制标准曲线[15],求回归方程和相关系数。
以实际养殖水体为研究对象进行标准添加实验,分别以低(0.5 μg/L)、中(5.0 μg/L)、高(20.0 μg/L)三个添加水平进行加标回收实验,每个浓度水平做6 次平行实验,考察方法的准确度及日内精密度,并连续6 d测定中等添加水平的加标回收率,考察方法的日间精密度。
1.8 计算公式
光降解率r计算公式:
式中,C0是水体中MBZ初始浓度,μg/L;Ct是时间t时水体中MBZ的残留浓度,μg/L。
2 结果与讨论
2.1 检测方法的实用性
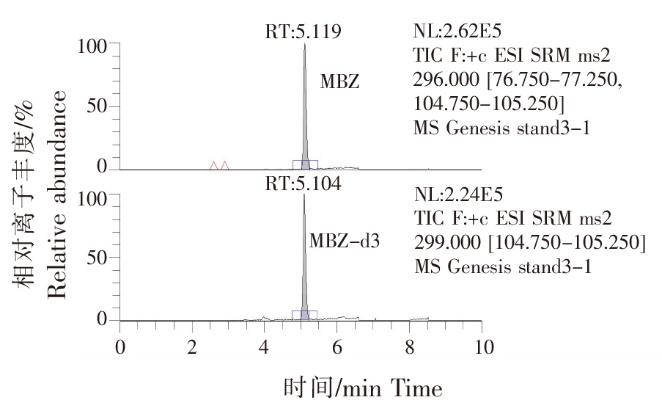
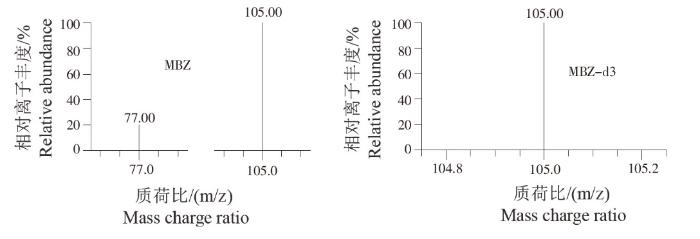
以10倍信噪比(S/N)计算定量限(Limit of quantitation,LOQ),养殖水体中 MBZ定量限为0.5 μg/L。在0.5~200 μg/L线性良好,标准曲线为:y=0.333 790x+0.243 444,R2>0.99。MBZ在0.5 μg/L添加水平下,回收率为70.6%~91.9%,日内变异系数在7.60%~8.63%之间;在5.0 μg/L添加水平下,回收率为70.8%~92.1%,日内变异系数在6.70%~9.65%之间;在20.0 μg/L添加水平下,回收率为84.5%~107%,日内变异系数在7.57%~8.27%之间。在3个浓度添加水平下,日间变异系数≤10%(表1)。MBZ定量和定性离子均无干扰,满足定量的要求。方法的精密度和准确度均能满足药物残留监测需求。标准品选择反应监测离子流色谱图见图2,标准品质谱图见图3。
表1 方法回收率和精密度测定结果
Tab.1
| 加标 浓度/(μg/L) Spiked concentration | 天数/d Days | 回收率/% Recoveries (n=6) | 日内精密度 Intra-day precision | 日间精密度 Inter-day precision | ||
|---|---|---|---|---|---|---|
| 平均回收率/% Average recovery (n=6) | 日内相对标 准偏差/% RSD(n=6) | 平均回收率/% Average recovery (n=18) | 日间相对标 准偏差/% RSD(n=18) | |||
| 0.5 | 1 | 73.6、77.1、85.6、90.8、82.4、87.0 | 82.8 | 7.77 | 81.9 | 7.97 |
| 5 | 82.4、88.6、83.1、70.6、73.9、75.1 | 79.0 | 8.63 | |||
| 9 | 89.7、82.5、85.9、91.9、77.4、76.3 | 84.0 | 7.60 | |||
| 5.0 | 1 | 70.8、83.5、88.6、79.6、87.4、71.4 | 80.2 | 9.65 | 83.3 | 8.11 |
| 5 | 86.1、86.2、79.1、77.9、90.2、92.0 | 85.3 | 6.70 | |||
| 9 | 90.4、81.8、87.6、80.4、74.9、92.1 | 84.5 | 7.93 | |||
| 20.0 | 1 | 90.5、107、91.3、88.4、84.8、93.6 | 92.6 | 8.27 | 93.3 | 7.43 |
| 5 | 84.5、88.6、90.1、96.1、101、103 | 93.9 | 7.81 | |||
| 9 | 90.1、93.5、88.8、105、85.4、97.8 | 93.4 | 7.57 | |||
图2
图2
标准品选择反应监测离子流色谱图(5 ug/L)
Fig.2
Selective reaction monitoring (SRM)chromatogram of mixed standards solution (5 ug/L)
图3
图3
标准品质谱图(5 ug/L)
Fig.3
Mass spectrometry of mixed standards solution (5 ug/L)
2.2 施药浓度的选择
水产养殖业中,外用杀虫剂MBZ的主要水产用剂型为10% MBZ溶液,施用途径为泼洒用药,具体用法为2 000倍水稀释均匀后泼洒。青鱼、草鱼、日本鳗鲡、鲢、鳙、鳜养殖水体每m水深用本品1 000~1 493 g/hm2 [16];欧洲鳗鲡、美洲鳗鲡养殖水体每m水深用本品2 493~4 985 g/hm2,若病情严重,第2 d再使用一次。青鱼、草鱼、日本鳗鲡、鲢、鳙、鳜养殖水体施药浓度换算成养殖水体浓度为 10~15 μg/L;欧洲鳗鲡、美洲鳗鲡养殖水体施药浓度换算成养殖水体浓度为 25~50 μg/L。根据实际养殖需求和条件,本实验选择了施药浓度为15 μg/L(低浓度组)、50 μg/L(高浓度组),单次泼洒,期间未换水,研究不同施药浓度下室外养殖水体中MBZ 的消除规律。
2.3 养殖水体中MBZ实际浓度
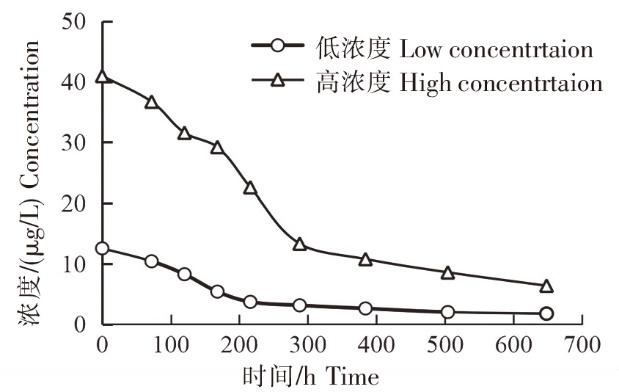
按照1.5、1.6 方法测定养殖水体中MBZ的消除规律,结果见表2。实验选择了施药浓度为15 μg/L(低浓度组)、50 μg/L(高浓度组),但实际养殖水体中MBZ浓度分别为12.6 μg/L(低浓度组)、40.0 μg/L(高浓度组),这与MBZ原粉在养殖水中的溶解度相关。1)MBZ溶解度随着水温的降低而降低。虽然实验采用与市售产品一样的助溶剂甲酸,但本实验水温较低,因此实际养殖水体中MBZ浓度低于理论值。2)养殖水体pH值也是影响MBZ溶解度的关键因素。当pH>8.5时,养殖水体会大量中和MBZ溶液,导致MBZ溶解性下降,从而降低MBZ的药效。由于MBZ的助溶剂为甲酸,因此本实验水体初始pH值约为6.8,但随着时间的推移,水体中的甲酸逐渐挥发,水体pH值逐渐增大,至实验结束时,pH值为7.5。该pH值范围不会影响MBZ的溶解性。在本实验条件下,养殖水体中MBZ实际浓度约为理论值的80%。
表2 养殖水体中 MBZ 药物实际浓度
Tab.2
| 采集时间/h Collection time | 浓度/(μg/L) Concentration | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 72 h | 120 h | 168 h | 216 h | 288 h | 384 h | 504 h | 648 h | |
| 低浓度组Low concentration group | 12.6 | 10.4 | 8.3 | 5.4 | 3.7 | 3.1 | 2.6 | 2.0 | 1.8 |
| 高浓度组High concentration group | 41.0 | 36.7 | 31.6 | 29.2 | 22.6 | 13.3 | 10.8 | 8.6 | 6.4 |
2.4 MBZ消除规律
光降解的效率取决于多种参数,如光的辐射强度、光照时长、药物结构、药物浓度等。在铝箔保护的对照组中,MBZ浓度没有随着时间的推移而下降,这说明实验期间的光解作用主要是由辐射引起的。如图4所示,随着时间的推移,前12 d(288 h),室外水体中MBZ在冬季自然光照下快速下降;此后MBZ消除速率明显变得缓慢。1)室外水体中MBZ的消除过程符合一级动力学方程 Ct =C0e
图4
实验结束(648 h)时,高浓度组养殖水体中仍有6.4 μg/L MBZ,约为青鱼等养殖品种水产养殖建议用药浓度的一半。这意味着,在实际养殖过程中,即自然光照条件下,若长时间未更换养殖水体,则其中的MBZ仍会有较多的残留,养殖动物也仍然会通过皮肤等组织持续吸收MBZ,从而造成养殖动物各组织中MBZ及其代谢产物的残留[19]。同样,在未及时更换养殖水体的情况下,MBZ溶液(水产用)建议的休药期将不适用于该水体的养殖动物。
3 结论
本研究系统地建立了高效液相色谱-串联质谱检测养殖水体中MBZ残留的方法,方法灵敏度高,准确度、精密度和各项技术指标均满足国内外残留检测的相关要求。同时在模拟的实际养殖条件下,以实际生产推荐使用浓度15、50 μg/L作为受试浓度,采用本文建立的检测方法,开展室外养殖水体中MBZ的消除实验,结果表明MBZ为光敏感药物,其降解规律遵循一级动力学。鉴于本实验条件下的研究结果,水温是影响养殖水体中MBZ消除的关键因素,因此建议当水温低于20℃时,应适当延长休药期;施药浓度也是影响养殖水体中MBZ消除的另一因素,当施药浓度为50 μg/L时,至实验结束(648 h),水体中仍有6.4 μg/L MBZ,因此建议施药7 d后,在达到药效的情况下,尽可能地更换水体,以防止养殖动物内MBZ的残留。由于长期暴露于MBZ下的养殖水体遵循动态消除规律,因此处于该养殖环境下的鱼类体内MBZ的残留更为复杂,需要进一步开展与鱼类相关的代谢动力学研究。
参考文献
One world health: socioeconomic burden and parasitic disease control priorities
[J].Parasitic diseases present a considerable socio-economic impact to society. Zoonotic parasites can result in a considerable burden of disease in people and substantive economic losses to livestock populations. Ameliorating the effects of these diseases may consist of attempts at eradicating specific diseases at a global level, eliminating them at a national or local level or controlling them to minimise incidence. Alternatively with some parasitic zoonoses it may only be possible to treat human and animal cases as they arise. The choice of approach will be determined by the potential effectiveness of a disease control programme, its cost and the cost effectiveness or cost benefit of undertaking the intervention. Furthermore human disease burden is being increasingly measured by egalitarian non-financial measures which are difficult to apply to livestock. This adds additional challenges to the assessment of socio-economic burdens of zoonotic diseases. Using examples from the group of neglected zoonotic diseases, information regarding the socio-economic effects is reviewed together with how this information is used in decision making with regard to disease control and treatment. Copyright © 2013 Elsevier B.V. All rights reserved.
Treatment of Microcotyle sebastis(Monogenea)on the gills of cultured rockfish (Sebastes schelegeli) with oral administration of mebendazole and bithionol
[J].
Treatment of Microcotyle sp.(Monogenea) on the gills of cage-cultured red porgy,Pagrus pagrus following baths with formalin and mebendazole
[J].
Effects of benzimidazole analogs on cultures of differentiating rodent embryonic cells
[J].Micromass cell culture systems for rat embryo midbrain (CNS) and limb bud (LB) cells were employed to assess the in vitro developmental toxicity of the benzimidazole analogs, mebendazole (MBZ), thiabendazole (TBZ), and nocodazole (NCZ), in addition to the classic microtubule inhibitor, colchicine. Comparison was made to albendazole (ABZ), a previously studied benzimidazole anthelmintic. Two parameters for assessing developmental toxicity were measured: differentiation and cytotoxicity. The relative potencies of the benzimidazole analogs in the micromass system (NCZ greater than MBZ approximately ABZ much greater than TBZ) mirrored their effectiveness in an assay for in vitro inhibition of mammalian tubulin polymerization. Colchicine also exhibits a high affinity for mammalian tubulin and was a potent inhibitor of cell proliferation, chondrogenesis, and neuronal differentiation. Immunofluorescent staining of Day 1 LB cultures with a monoclonal antibody to beta-tubulin revealed that these agents elicited mitotic arrest. Many anti-tubulin agents are teratogenic in rats and their in vivo developmental toxicity may reflect perturbation of microtubular structure or function. With the exception of TBZ, these agents should be considered potential developmental toxicants since they inhibit cell growth and differentiation of micromass cultures at nanomolar concentrations.
DNA damage response is involved in the developmental toxicity of mebendazole in zebrafish retina
[J].Intestinal helminths cause iron-deficiency anemia in pregnant women, associated with premature delivery, low birth weight, maternal ill health, and maternal death. Although benzimidazole compounds such as mebendazole (MBZ) are highly efficacious against helminths, there are limited data on its use during pregnancy. In this study, we performed in vivo imaging of the retinas of zebrafish larvae exposed to MBZ, and found that exposure to MBZ during 2 and 3 days post-fertilization caused malformation of the retinal layers. To identify the molecular mechanism underlying the developmental toxicity of MBZ, we performed transcriptome analysis of zebrafish eyes. The analysis revealed that the DNA damage response was involved in the developmental toxicity of MBZ. We were also able to demonstrate that inhibition of ATM significantly attenuated the apoptosis induced by MBZ in the zebrafish retina. These results suggest that MBZ causes developmental toxicity in the zebrafish retina at least partly by activating the DNA damage response, including ATM signaling, providing a potential adverse outcome pathway in the developmental toxicity of MBZ in mammals.
丁香酚在罗非鱼体内的代谢和休药期研究
[J].为探讨丁香酚在罗非鱼血浆和肌肉中的药代动力学特征及残留消除规律,在(25±1)℃水温条件下,以20 mg/L丁香酚溶液麻醉罗非鱼24 h后,按时间段分别采集血浆和肌肉样品,测定罗非鱼血浆和肌肉中的丁香酚残留,确定丁香酚在罗非鱼体内的药代动力学参数及代谢过程。结果显示,罗非鱼血浆和肌肉中的药物经时过程均符合一级吸收一室开放模型,其理论方程分别为C血浆=4.762(e-0.062 6t-e-0.064 7t)、C肌肉=1.089(e-0.006 9t- e-0.0021 7t)。血浆中的主要药动学参数:T1/2ka为14.06 h; T1/2ke为14.68 h; Cmax为5.18 mg/kg。肌肉中的主要药动学参数:T1/2ka为84.80 h; T1/2ke为101.48 h; Cmax为3.07 mg/kg,可见丁香酚在罗非鱼血浆中的吸收、消除速度均明显快于肌肉。结果表明,以0.05 mg/kg为最高残留限量计算,在本试验条件下,以肌肉为残留检测的靶组织,丁香酚在罗非鱼体内的休药期不低于12 d,因此,不建议丁香酚作为罗非鱼运输的麻醉剂使用。
分散固相萃取-高效液相色谱串联质谱法同时测定沉积物中氟喹诺酮类药物残留
[J].本文建立了分散固相萃取结合高效液相色谱-串联质谱同时测定沉积物中氟喹诺酮类药物残留的分析方法。沉积物经20 mL乙腈-磷酸盐缓冲液(1:1,V/V)超声提取,0.15 g乙二胺四乙酸二钠络合除杂,分散固相萃取材料净化,Ultimate XB-C18色谱柱分离,含4 mmol/L乙酸铵的0.1%甲酸水溶液-0.1%甲酸甲醇梯度洗脱,电喷雾正离子模式下以多反应监测方式检测,内标法定量分析。通过试验优化了不同提取方法、净化吸附剂比。结果表明,5种氟喹诺酮类药物在2.5~200 ng/mL范围内线性关系良好(R2>0. 99),方法定量限(S/N≥10) 为2 μg/kg。在2~50 μg/kg 添加水平内,平均回收率为79.8%~112%,日内相对标准偏差(RSD)为3.2%~9.9%,日间RSD为5.3%~8.6%。
Behavior of mebendazole during NF/RO adsorption and photolysis
[J].The idea of using drugs from the benzimidazole group as potential antitumor agents is becoming increasingly popular and widespread in research. However, their use as antiparasitics and in cancer treatment will increase their already recorded occurrence in the aquatic environment. In this study, the removal of the anthelmintic mebendazole from aqueous solution was investigated using nanofiltration and reverse osmosis membranes, adsorption on granular activated carbon (GAC), and photolytic degradation. The dense NF90 and reverse osmosis XLE membranes showed almost complete removal (>97.7%), while the NF270 membrane showed a large dependence of removal on initial concentration from 41.9% to 96.6%. Adsorption in the column resulted in complete removal of mebendazole at the highest GAC height used (40 cm) from the solution with the lowest concentration (1 mg/L). Photolytic degradation by artificial light for 2 and 12 h resulted in photodegradation of mebendazole in the range of 23.5–61.4%, forming a new degradation or transformation compound with an m/z ratio of 311. Mebendazole is a photosensitive drug whose photodegradation follows first-order kinetics and depends on the drug concentration. Toxicity was studied with Vibrio fischeri before and after photolysis, and showed a decrease in inhibition after 12 h.