趋化因子是一种趋化性细胞因子,在炎症和生理条件下对细胞运动和激活起重要作用[1⇓-3]。趋化因子超家族也是连接先天性免疫和获得性免疫的重要桥梁[4-5]。它们参与了脊椎动物神经发育、血管生成、器官生成、生殖细胞迁移和缺氧反应等过程[6⇓⇓⇓⇓⇓⇓-13]。趋化因子受体是一种G蛋白偶联受体,包含了7个跨膜结构[14],根据受体N-端附近半胱氨酸残基的间距,可以将这些受体分为4个亚家族(CXC、CC、CX3C和XC)[15]。同时,趋化因子超家族包括大量的配体,大多数趋化因子可与多个受体结合,一个受体也可与多个趋化因子结合[16]。虽然趋化因子及其受体在哺乳动物,尤其是人类中得到了广泛的研究,但趋化因子受体在硬骨鱼尤其在鱼类免疫中的作用尚不明确。
斜带石斑鱼(Epinephelus coioides),属于鲈形目(Perciformes)、鮨科(Serranidae)、石斑鱼亚科(Epinephelinae)、石斑鱼属(Epinephelus),为广盐性暖水性中下层鱼类,主要分布于太平洋和印度洋的热带及亚热带海区。石斑鱼是中国重要的海洋经济鱼类养殖品种。然而,近年来各种病毒性疾病的暴发影响了石斑鱼养殖的发展[23],尤其是赤点石斑鱼神经坏死病毒(RGNNV),受其感染会导致超过90%的鱼苗死亡。本研究克隆了斜带石斑鱼CCR6a基因ORF序列,并分析了该基因的进化关系,通过基因过表达和RT-qPCR方法研究了该基因组织分布、亚细胞定位和抗病毒功能,发现CCR6a可显著抑制RGNNV的复制。由于CCR6在抗病毒免疫反应中发挥着重要作用,因此研究CCR6与抗RGNNV之间的关系将有助于防治该病毒病。
1 材料与方法
1.1 实验材料
1.1.1 实验动物
本实验所用斜带石斑鱼来自广东省阳江市广东海元农业科技有限公司养殖基地,挑取3尾体质量在500~750 g之间的健康鱼,取样前对鱼进行麻醉处理,取肝、脾、垂体、头肾、下丘脑、肾、性腺、肌肉、鳃、肠、皮肤和心脏组织于液氮中速冻,置于-80℃超低温冰箱中保存,用于RNA提取、基因克隆和组织表达研究。
1.1.2 细胞实验
研究中使用的石斑鱼脾脏(GS)细胞是本实验室前期培育的石斑鱼脾脏细胞系。RGNNV是实验室前期分离、培养和保存的病毒。用RGNNV感染GS细胞一定时间,在感染后的特定时间点,收集病毒感染的细胞并进行分析。
1.2 CCR6a基因ORF区域的克隆和分析
1.2.1 总RNA提取
采用 Trizol reagent 说明书及操作步骤来提取总 RNA,采用cDNA合成试剂盒(罗氏)反转录成 cDNA 模板,用于基因克隆和实时定量 PCR。
1.2.2 引物设计
从斜带石斑鱼基因组数据库中获取CCR6a核心序列信息,结合NCBI数据库获得已报道的鱼类CCR6基因序列进行分析,使用Primer 5.0在线引物搜索工具设计CCR6a基因ORF区域引物,引物序列为CCR6a-F: GATGAACTACACCAACCAATC,CCR6a-R: CCTCACATGGTGAAAGATGAGC (表1)。
表1 CCR6a基因克隆和RT-qPCR所用引物
Tab.1
| 引物名称Primer name | 引物序列(5’-3’) Primer sequences (5’-3’) |
|---|---|
| CCR6a-F | GATGAACTACACCAACCAATC |
| CCR6a-R | CCTCACATGGTGAAAGATGAGC |
| pEGFP-C1-1F | CCGGAATTCTATGAACTACACCAACCAATCAG(ECORI) |
| pEGFP-C1-1R | CGGGGTACCCATGGTGAAAGATGAGCCGTTTTC(KPNI) |
| qCCR6a-F | GATCGTCACCTACGCCTTCTAC |
| qCCR6a-R | GCTACATCGCCATTGTCCAG |
| qCP-F | CAACTGACAACGATCACACCTTC |
| qCP-R | CAATCGAACACTCCAGCGACA |
| qRdRp-F | GTGTCCGGAGAGGTTAAGGATG |
| qRdRp-R | CTTGAATTGATCAACGGTGAACA |
| β Actin-F | TACGAGCTGCCTGACGGACA |
| β Actin-R | GGCTGTGATCTCCTTCTGCA |
1.2.3 CCR6a 基因的克隆与分析
用反转录出来的cDNA为模板,采用设计的引物进行聚合酶链式反应来克隆CCR6a的ORF序列。琼脂糖凝胶电泳验证后,用胶回收试剂盒 (Omega Bio-Tek,USA) 回收目的条带,对回收产物进行连接转化和菌液验证,将验证后的菌液送至擎科生物科技有限公司进行测序。测序结果出来后,CCR6a的氨基酸序列利用 BioEdit 软件翻译,CCR6a蛋白序列的多重比对和进化树分析使用ClustalW 1.83和MEGA 5.0软件进行。
1.2.4 表达载体的构建
将CCR6a亚克隆到pEGFP-C1表达载体中,研究CCR6a在石斑鱼GS细胞中的定位和潜在功能。pEGFP-C1表达载体购买自Takara公司(货号:PT3028-5),GS细胞来自本实验室前期构建的石斑鱼脾脏细胞系,质粒构建的引物如表1所示,pEGFP-C1-1F:CCGGAATTCTATGAACTACACCAACCAATCAG,pEGFP-C1-1R:CGGGGTACCCATGGTGAAAGATGAGCCGTTTTC。采用双酶切方法将CCR6a的ORF区连接到载体,采用的两种酶是ECORI和KPNI,酶切位点在引物序列中标粗,重组质粒经DNA测序证实。
1.2.5 细胞定位分析
1×105GS细胞加入6孔板中,培养24 h后,采用Invitrogen 公司的Lipofectamine 2000 (货号:11668-019)转染试剂将pEGFP-C1和pEGFP-CCR6a质粒转染入GS细胞中,继续培养48 h后,用4%多聚甲醛固定细胞,然后用1 mg/mL DAPI染核10 min,最后用PBS冲洗细胞,50%甘油固定,荧光显微镜观察CCR6a的定位情况。
1.2.6 病毒感染实验
本研究中使用的石斑鱼GS细胞培养在添加10%胎牛血清(FBS)的Leibovitz’ L 15培养基中,培养24 h后将细胞传到24孔板中,24 h后将pEGFP-C1和pEGFP-CCR6a质粒转染到GS细胞中,转染完后12 h,用RGNNV感染GS细胞24 h,再收集病毒感染的细胞,提取RNA,通过实时荧光定量分析病毒基因的表达量。
1.2.7 实时定量 PCR 及数据分析
使用SYBR Green I Master(罗氏)在罗氏LightCycler 480实时PCR系统上进行实时PCR分析。PCR条件如下:95℃活化5 min,然后在95℃ 20 s、58℃ 20 s和72℃ 20 s下循环40个周期。定量引物如表1所示,其中引物qCP-F/R表示RGNNV衣壳蛋白基因(Capsid protein, CP)定量引物,qRdRp-F/R 表示RGNNV RNA聚合酶基因(RNA-dependent RNA polymerase,RdRp),以β-actin基因作为内参,表1中β Actin-F/R所示为内参引物。分析溶解曲线,确定RT-qPCR的质量,用Excel表格初步处理数据,再利用Graphpad Prism 5.0软件对数据进行处理和分析。
2 结果与分析
2.1 斜带石斑鱼CCR6a基因克隆及其序列分析
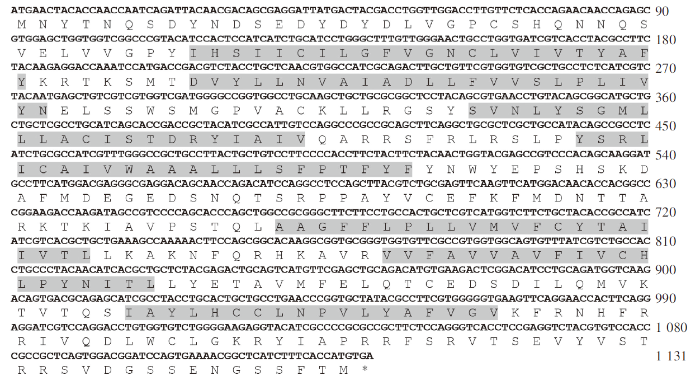
测序结果表明CCR6a包含ORF的cDNA序列,ORF长为1 131 bp,编码376个氨基酸(图1) 。用TMHMM Server V.2.0分析得到7个跨膜区域(Transmembrane domain,TM),分别为TM1(AA39~61)、TM2(AA70~92)、TM3(AA112~134)、TM4(AA147~169)、TM5(AA223~245)、TM6(AA258~277)和TM7(AA306~323)。
图1
图1
斜带石斑鱼CCR6a 基因的cDNA 序列及其推导出的氨基酸序列
注: 阴影表示七次跨膜区域,*表示终止密码子。
Fig.1
The nucleotide and deduced amino acid sequences of CCR6a
Notes: The shadow indicated the seven-degree transmembrane region, and *indicated the termination codon.
NetPhos 2.0 Server预测CCR6a氨基酸序列有36个磷酸化位点、17个丝氨酸磷酸化位点、12个苏氨酸磷酸化位点和7个酪氨酸磷酸化位点。NetNGlyc 1.0 Server预测CCR6a氨基酸序列有8个糖基化位点,其中有1个位于跨膜结构区域内(AA274),1个位于胞内的C末端区域(AA370)。
2.2 CCR6a氨基酸序列的系统进化树分析
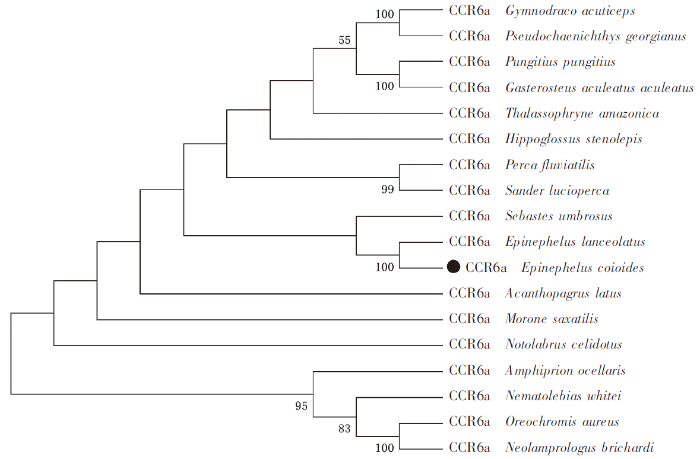
进化树分析结果显示,斜带石斑鱼CCR6a基因与硬骨鱼类的CCR6a基因聚为一支,进一步分析发现 CCR6a与鞍带石斑鱼(Epinephelus lanceolatus)的CCR6a聚为一支(图2)。进化树中选取的相关物种及其基因在 GenBank 的序列号构建进化树所用的基因的序列号如下: CCR6a Epinephelus lanceolatus,XP_033504450.1;CCR6a Perca fluviatilis,XP_039637708.1;CCR6a Sebastes umbrosus,XP_037605863.1;CCR6a Acanthopagrus latus,XP_036943380.1;CCR6a Hippoglossus stenolepis,XP_035006979.1;CCR6a Morone saxatilis,XP_035534502.1;CCR6a Sander lucioperca,XP_031171690.1;CCR6a Gymnodraco acuticeps,XP_034057877.1;CCR6a Amphiprion ocellaris,XP_023129801.1;CCR6a Pungitius pungitius,XP_037305948.1;CCR6a Thalassophryne amazonica,XP_034018486.1;CCR6a Pseudochaenichthys georgianus,XP_033935746.1;CCR6a Notolabrus celidotus,XP_034555014.1;CCR6a Gasterosteus aculeatus aculeatus,XP_040025791.1;CCR6a Oreochromis aureus,XP_031603377.2;CCR6a Neolamprologus brichardi,XP_006793517.1;CCR6a CCR6a Nematolebias whitei,XP_037541943.1。
图2
图2
趋化因子受体CCR6a 蛋白质进化树分析
注:斜带石斑鱼CCR6a氨基酸序列与其他物种的同源序列构建的系统进化树。进化树通过MEGA-X的邻接(N-J)法构建,以最大似然法重复1 000次。
Fig.2
Analysis of the evolutionary tree of the chemokine receptor CCR6a protein
Notes: Phylogenetic tree using CCR6a amino acid sequences of orange-spotted grouper and homologous sequences of other species.The tree was constructed by the neighbor-joining method of MEGA-X and repeated 1 000 times by the maximum likelihood method.
2.3 CCR6a基因在斜带石斑鱼不同组织中的表达
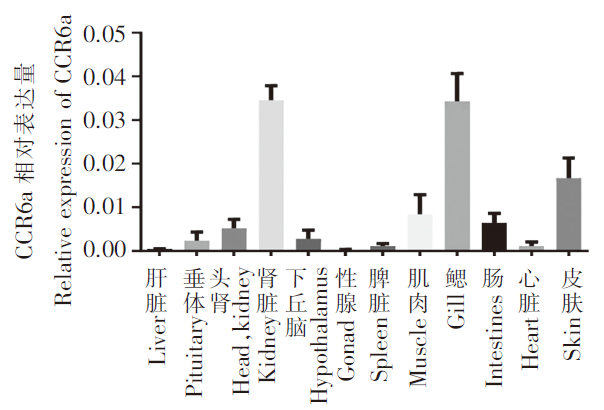
通过定量PCR检测CCR6a在不同组织中的表达水平,结果如图3所示,CCR6a基因在斜带石斑鱼的肾脏和鳃中表达量较高,皮肤次之,性腺和肝脏中表达较少。从中可看出,CCR6a基因在免疫组织和非免疫组织的表达水平表现出明显差异,其在免疫组织中的表达水平远高于非免疫组织。
图3
图3
趋化因子受体CCR6a 在斜带石斑鱼不同组织的相对表达量
Fig.3
Relative expression of the chemokine receptor CCR6a in different tissues of oblique grouper
2.4 CCR6a基因在细胞中的定位
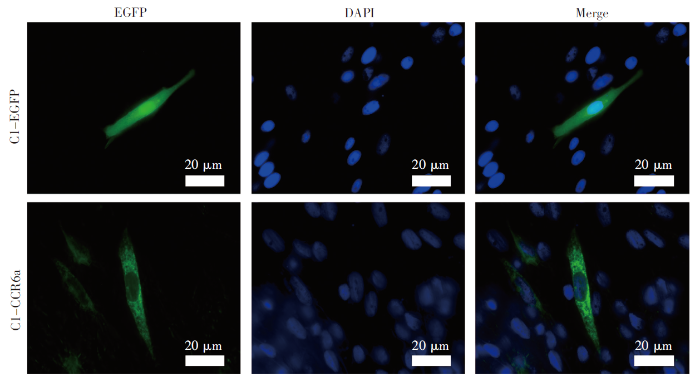
构建斜带石斑鱼CCR6a基因的pEGFP-C1载体,通过转染,将带有EGFP标签的CCR6a融合蛋白转染到GS细胞中,以pEGFP-C1的空载体作为对照,在荧光显微镜下观察,结果表明pEGFP-C1转染GS后表达的EGFP在细胞核和细胞质中均有分布;而pEGFP-CCR6a转染的GS细胞中表达的CCR6a存在于细胞的膜系统中(图4)。
图4
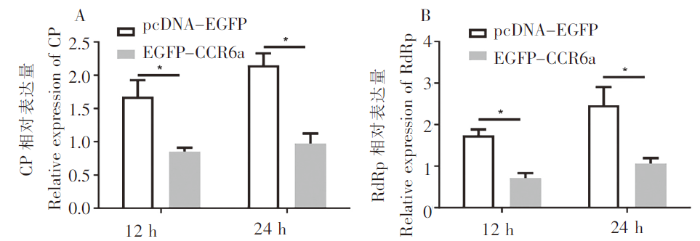
2.5 体外验证CCR6a基因抗病毒能力
图5
图5
在CCR6a 过表达细胞中的病毒基因表达情况
Fig.5
Viral gene transcription of RGNNV in CCR6a overexpressing cells
3 讨论
本研究通过克隆获得斜带石斑鱼CCR6a基因ORF序列,其序列全长为1 131 bp,编码376个氨基酸,通过TMHMM Server V.2.0预测含有7个跨膜结构域,具有G蛋白偶联受体的典型特征。斜带石斑鱼CCR6a序列与其他物种同源性分析发现,斜带石斑鱼CCR6a与大多数鱼类的同源性比较高。系统进化树分析表明斜带石斑鱼CCR6a与鞍带石斑鱼聚为一支。组织表达分析显示,CCR6a在肾脏、鳃和皮肤等免疫组织中有较高的表达。在斑马鱼(Danio rerio)中,CCR主要在大脑和免疫系统中表达[24];在大西洋鲑(Salmo salar)中,CCR在脾脏和鳃中表达最高[25];在虹鳟(Oncorhynchus mykiss)中,CCR表达水平最高的部位是胸腺和脾脏[26],本研究发现的CCR6a在肾脏、鳃和皮肤中高表达,与其他鱼类中关于CCR在免疫器官高表达结果一致。这些研究结果表明,鱼类CCR可能在免疫调节中发挥重要作用。近年来趋化因子受体在脊椎动物免疫应答中的作用备受关注[25,27],已有许多研究表明趋化因子受体CCR6与机体的各类疾病有着密切的联系[28⇓⇓-31],但是关于趋化因子在鱼类中的免疫反应,尤其是对病原入侵中的作用知之甚少,因此了解趋化因子受体在鱼类病原体防御中的作用至关重要。
在鱼类中,有研究表明CCR6a对抵抗细菌和寄生虫的感染有重要作用,在大菱鲆(Scophthalmus maximus)中,CCR6a对减少杀鲑气单胞菌(Aeromonas salmonicida)感染有重要作用[32],在斜带石斑鱼中发现CCR6可能在刺激隐核虫引起的炎症时免疫细胞向皮肤黏膜免疫组织的归化过程中发挥重要作用[33]。前期研究表明CCR在细菌和寄生虫感染中起着重要作用,而CCR6a作为CCR家族中的重要成员,在病毒感染中也起着重要作用。在人类中,有研究表明CCR6作为一种新的辅受体参与HIV和SIV毒株的入侵[34],此外CCR6/CCL20趋化因子轴在HIV发病和免疫中发挥重要作用[35]。在鱼类中还没有CCR6与病毒入侵和抗病毒方面的研究,但是有大量研究表明趋化因子家族对鱼类病毒的入侵和抗病毒免疫反应的初始阶段起着重要作用[36⇓-38]。本研究发现在石斑鱼中CCR6a过表达显著抑制RGNNV复制,CCR6a作为膜蛋白可能阻止了病毒的入侵,从而降低病毒在细胞内的总量,也有可能如之前的研究发现,CCR可以通过诱导石斑鱼细胞中IFN调控因子显著增加和提高ISRE、IFN启动子活性,从而提高干扰素生产来抵抗病毒[39],但是这需要进一步的研究证实。总体来说,本研究结果提示CCR6a可能是鱼类重要的抗病毒因子之一。
4 结论
本研究从斜带石斑鱼组织中克隆出了CCR6a基因的ORF序列,并对其序列和进化关系进行了分析,同时研究了其组织分布、亚细胞定位和抗病毒情况。结果表明,斜带石斑鱼CCR6a基因ORF序列全长为1 131 bp,编码376个氨基酸;进化树分析发现,斜带石斑鱼CCR6a与同为石斑鱼属的鞍带石斑鱼聚为一支;组织分布研究表明,斜带石斑鱼CCR6a在免疫组织中的表达水平显著高于非免疫组织;利用荧光显微镜对其亚细胞定位分析表明,该基因为膜分布;此外,过表达显著抑制了体外RGNNV的复制。研究结果将有助于理解鱼类趋化因子在病毒感染中的作用。
参考文献
Evolution of CC chemokines in teleost fish: a case study in gene duplication and implications for immune diversity
[J].Chemokines are a superfamily of cytokines responsible for regulating cell migration under both inflammatory and physiological conditions. CC chemokines are the largest subfamily of chemokines, with 28 members in humans. A subject of intense study in mammalian species, the known functional roles of CC chemokines ligands in both developmental and disease conditions continue to expand. They are also an important family for the study of gene copy number variation and tandem duplication in mammalian species. However, little is known regarding the evolutionary origin and status of these ligands in primitive vertebrates such as teleost fish. In this paper, we review the evolution of the teleost fish CC chemokine gene family, noting evidence of widespread tandem gene duplications and examining the implications of this phenomenon on immune diversity. Through extensive phylogenetic analysis of the CC chemokine sets of four teleost species, zebrafish, catfish, rainbow trout, and Atlantic salmon, we identified seven large groups of CC chemokines. It appeared that several major groups of CC chemokines are highly related including the CCL19/21/25 group, the CCL20 group, CCL27/28 group, and the fish-specific group. In the three remaining groups that contained the largest number of members, the CCL17/22 group, the MIP group, and the MCP group, similarities among species members were obscured by rapid, tandem duplications that may contribute to immune diversity.
Extensive expansion and diversification of the chemokine gene family in zebrafish: identification of a novel chemokine subfamily CX
[J].The chemokine family plays important roles in cell migration and activation. In humans, at least 44 members are known. Based on the arrangement of the four conserved cysteine residues, chemokines are now classified into four subfamilies, CXC, CC, XC and CX3C. Given that zebrafish is an important experimental model and teleost fishes constitute an evolutionarily diverse group that forms half the vertebrate species, it would be useful to compare the zebrafish chemokine system with those of mammals. Prior to this study, however, only incomplete lists of the zebrafish chemokine genes were reported.We systematically searched chemokine genes in the zebrafish genome and EST databases, and identified more than 100 chemokine genes. These genes were CXC, CC and XC subfamily members, while no CX3C gene was identified. We also searched chemokine genes in pufferfish fugu and Tetraodon, and found only 18 chemokine genes in each species. The majority of the identified chemokine genes are unique to zebrafish or teleost fishes. However, several groups of chemokines are moderately similar to human chemokines, and some chemokines are orthologous to human homeostatic chemokines CXCL12 and CXCL14. Zebrafish also possesses a novel species-specific subfamily consisting of five members, which we term the CX subfamily. The CX chemokines lack one of the two N-terminus conserved cysteine residues but retain the third and the fourth ones. (Note that the XC subfamily only retains the second and fourth of the signature cysteines residues.) Phylogenetic analysis and genome organization of the chemokine genes showed that successive tandem duplication events generated the CX genes from the CC subfamily. Recombinant CXL-chr24a, one of the CX subfamily members on chromosome 24, showed marked chemotactic activity for carp leukocytes. The mRNA was expressed mainly during a certain period of the embryogenesis, suggesting its role in the zebrafish development.The phylogenic and genomic organization analyses suggest that a substantial number of chemokine genes in zebrafish were generated by zebrafish-specific tandem duplication events. During such duplications, a novel chemokine subfamily termed CX was generated in zebrafish. Only two human chemokines CXCL12 and CXCL14 have the orthologous chemokines in zebrafish. The diversification observed in the numbers and sequences of chemokines in the fish may reflect the adaptation of the individual species to their respective biological environment.
Chemokines: a new classification system and their role in immunity
[J].
Chemokines in health and disease
[J].Chemokines and their receptors play a key role in development and homeostasis as well as in the pathogenesis of tumors and autoimmune diseases. Chemokines are involved in the implantation of the early conceptus, the migration of subsets of cells during embryonic development, and the overall growth of the embryo. Chemokines also have an important role in the development and maintenance of innate and adaptive immunity. In addition, they play a significant role in wound healing and angiogenesis. When the physiological role of chemokines is subverted or chronically amplified, disease often follows. Chemokines are involved in the pathobiology of chronic inflammation, tumorigenesis and metastasis, as well as autoimmune diseases. This article reviews the role of chemokines and their receptors in normal and disease processes and the potential for using chemokine antagonists for appropriate targeted therapy.Published by Elsevier Inc.
Chemokines in teleost fish species
[J].Chemokines are chemoattractant cytokines defined by the presence of four conserved cysteine residues which in mammals can be divided into four subfamilies depending on the arrangement of the first two conserved cysteines in their sequence: CXC (α), CC (β), C and CX(3)C classes. Evolutionarily, fish can be considered as an intermediate step between species which possess only innate immunity (invertebrates) and species with a fully developed acquired immune network such as mammals. Therefore, the functionality of their different immune cell types and molecules is sometimes also intermediate between innate and acquired responses. The first chemokine gene identified in a teleost was a rainbow trout (Oncorhynchus mykiss) chemokine designated as CK1 in 1998. Since then, many different chemokine genes have been identified in several fish species, but their role in homeostasis and immune response remains largely unknown. Extensive genomic duplication events and the fact that chemokines evolve more quickly than other immune genes, make it very difficult to establish true orthologues between fish and mammalian chemokines that would help us with the ascription of immune roles. In this review, we describe the current state of knowledge of chemokine biology in teleost fish, focusing mainly on which genes have been identified so far and highlighting the most important aspects of their expression regulation, due to the great lack of functional information available for them. As the number of chemokine genes begins to close down for some teleost species, there is an important need for functional assays that may elucidate the role of each of these molecules within the fish immune response.Copyright © 2011 Elsevier Ltd. All rights reserved.
A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor
[J].
The role of CXC chemokines in the regulation of angiogenesis in non-small cell lung cancer
[J].Angiogenesis is a critical component of tumor biology. In recent years newer techniques of cell and molecular biology have led to important advances in our understanding of this process. The regulation of angiogenesis depends on a balance between the activity of local factors that promote (angiogenic factors) or inhibit (angiostatic factors) neovascularization. Nowhere is this paradigm of a balance more apparent than in the study of tumor-associated angiogenesis. Tumors promote angiogenesis through a combination of overexpression of angiogenic factors and local inhibition of angiostatic factors. This strategy leads to an angiogenic environment that promotes tumor growth and metastases. Our laboratory has focused studies on the role of the CXC chemokine family in the regulation of angiogenesis by non-small cell lung cancer (NSCLC). In this article, we review our findings that the CXC chemokine family is composed of members that are either angiogenic or angiostatic. We have found that in NSCLC an imbalance exists in the expression of these factors that favors tumor-derived angiogenesis, and therefore tumor growth and metastases. Furthermore, when this imbalance is corrected to reduce the presence of angiogenic factors or increase the presence of angiostatic factors, tumor growth and metastases are reduced.
Chemokines regulate the migration of neural progenitors to sites of neuroinflammation
[J].Many studies have shown that transplanted or endogenous neural progenitor cells will migrate toward damaged areas of the brain. However, the mechanism underlying this effect is not clear. Here we report that, using hippocampal slice cultures, grafted neural progenitor cells (NPs) migrate toward areas of neuroinflammation and that chemokines are a major regulator of this process. Migration of NPs was observed after injecting an inflammatory stimulus into the area of the fimbria and transplanting enhanced green fluorescent protein (EGFP)-labeled NPs into the dentate gyrus of cultured hippocampal slices. Three to 7 d after transplantation, EGFP-NPs in control slices showed little tendency to migrate and had differentiated into neurons and glia. In contrast, in slices injected with inflammatory stimuli, EGFP-NPs migrated toward the site of the injection. NPs in these slices also survived less well. The inflammatory stimuli used were a combination of the cytokines tumor necrosis factor-alpha and interferon-gamma, the bacterial toxin lipopolysaccharide, the human immunodeficiency virus-1 coat protein glycoprotein 120, or a beta-amyloid-expressing adenovirus. We showed that these inflammatory stimuli increased the synthesis of numerous chemokines and cytokines by hippocampal slices. When EGFP-NPs from CC chemokine receptor CCR2 knock-out mice were transplanted into slices, they exhibited little migration toward sites of inflammation. Similarly, wild-type EGFP-NPs exhibited little migration toward inflammatory sites when transplanted into slices prepared from monocyte chemoattractant protein-1 (MCP-1) knock-out mice. These data indicate that factors secreted by sites of neuroinflammation are attractive to neural progenitors and suggest that chemokines such as MCP-1 play an important role in this process.
Chemokines direct neural progenitor cell migration following striatal cell loss
[J].In this study we demonstrate the chemokines MCP-1, MIP-1alpha and GRO-alpha play a role in directing adult subventricular zone (SVZ)-derived progenitor cell migration following striatal cell death. MCP-1, MIP-1alpha and GRO-alpha were significantly upregulated in the striatum 2-3 days following QA-induced lesioning, correlating with maximum SVZ-derived progenitor cell recruitment into the lesioned striatum. We established that SVZ-derived progenitor cells express receptors for each chemokine, and demonstrated MCP-1, MIP-1alpha and GRO-alpha to be potent chemoattractants for SVZ-derived progenitor cells in vitro. Immunofluorescence revealed MCP-1, MIP-1alpha and GRO-alpha are predominantly expressed in the striatum by NG2-positive cells that appear to infiltrate from the bloodstream 6 h following QA lesioning. These results indicate that upregulation of MCP-1, MIP-1alpha and GRO-alpha following striatal cell death leads to chemoattraction of SVZ-derived progenitor cells into the damaged striatum and raises a potential role for blood-derived cells in directing the recruitment of SVZ-derived progenitors following brain injury.
CXC chemokines and angiogenesis/angiostasis
[J].Angiogenesis is important to a variety of physiological and pathological processes. While a variety of factors have been determined to regulate angiogenesis, members of the CXC chemokine family can either promote or inhibit this process. This disparity in biological behavior is due to the presence or absence of a structural-functional domain--three amino acid residues (Glu-Leu-Arg: the "ELR-motif") that precede the first cysteine amino acid residue of the primary structure of these cytokines. The purpose of this study is to introduce the topic of angiogenesis and focuses on the CXC chemokine family, because these cytokines are a unique family of molecules that can behave in a disparate manner in the regulation of angiogenesis associated with either chronic inflammatory-fibroproliferative disorders or tumor growth.
Guidance of primordial germ cell migration by the chemokine SDF-1
[J].The signals directing primordial germ cell (PGC) migration in vertebrates are largely unknown. We demonstrate that sdf-1 mRNA is expressed in locations where PGCs are found and toward which they migrate in wild-type as well as in mutant embryos in which PGC migration is abnormal. Knocking down SDF-1 or its receptor CXCR4 results in severe defects in PGC migration. Specifically, PGCs that do not receive the SDF-1 signal exhibit lack of directional movement toward their target and arrive at ectopic positions within the embryo. Finally, we show that the PGCs can be attracted toward an ectopic source of the chemokine, strongly suggesting that this molecule provides a key directional cue for the PGCs.
Hypoxia modifies the transcriptome of primary human monocytes: modulation of novel immune-related genes and identification of CC-chemokine ligand 20 as a new hypoxia-inducible gene
[J].Peripheral blood monocytes migrate to and accumulate in hypoxic areas of inflammatory and tumor lesions. To characterize the molecular bases underlying monocyte functions within a hypoxic microenvironment, we investigated the transcriptional profile induced by hypoxia in primary human monocytes using high-density oligonucleotide microarrays. Profound changes in the gene expression pattern were detected following 16 h exposure to 1% O(2), with 536 and 677 sequences showing at least a 1.5-fold increase and decrease, respectively. Validation of this analysis was provided by quantitative RT-PCR confirmation of expression differences of selected genes. Among modulated genes, 74 were known hypoxia-responsive genes, whereas the majority were new genes whose responsiveness to hypoxia had not been previously described. The hypoxic transcriptome was characterized by the modulation of a significant cluster of genes with immunological relevance. These included scavenger receptors (CD163, STAB1, C1qR1, MSR1, MARCO, TLR7), immunoregulatory, costimulatory, and adhesion molecules (CD32, CD64, CD69, CD89, CMRF-35H, ITGB5, LAIR1, LIR9), chemokines/cytokines and receptors (CCL23, CCL15, CCL8, CCR1, CCR2, RDC1, IL-23A, IL-6ST). Furthermore, we provided conclusive evidence of hypoxic induction of CCL20, a chemoattractant for immature dendritic cells, activated/memory T lymphocytes, and naive B cells. CCL20 mRNA up-regulation was paralleled by increased protein expression and secretion. This study represents the first transcriptome analysis of hypoxic primary human monocytes, which provides novel insights into monocyte functional behavior within ischemic/hypoxic tissues. CCL20 up-regulation by hypoxia may constitute an important mechanism to promote recruitment of specific leukocyte subsets at pathological sites and may have implications for the pathogenesis of chronic inflammatory diseases.
Regulation of the chemokine receptor CXCR4 by hypoxia
[J].Cell adaptation to hypoxia (Hyp) requires activation of transcriptional programs that coordinate expression of genes involved in oxygen delivery (via angiogenesis) and metabolic adaptation (via glycolysis). Here, we describe that oxygen availability is a determinant parameter in the setting of chemotactic responsiveness to stromal-derived factor 1 (CXCL12). Low oxygen concentration induces high expression of the CXCL12 receptor, CXC receptor 4 (CXCR4), in different cell types (monocytes, monocyte-derived macrophages, tumor-associated macrophages, endothelial cells, and cancer cells), which is paralleled by increased chemotactic responsiveness to its specific ligand. CXCR4 induction by Hyp is dependent on both activation of the Hyp-inducible factor 1 α and transcript stabilization. In a relay multistep navigation process, the Hyp–Hyp-inducible factor 1 α–CXCR4 pathway may regulate trafficking in and out of hypoxic tissue microenvironments.
Chemokines, chemokine receptors, and cancer metastasis
[J].It is clear from large clinical studies that selected chemokine receptors are often up-regulated in a large number of common human cancers, including those of the breast, lung, prostate, colon, and melanoma. Chemokine receptors and their corresponding chemokine ligands have been demonstrated to play a number of nonredundant roles in cancer metastasis to vital organs as well as regional lymph nodes, the most frequent site of cancer metastasis. Chemokine receptors may potentially facilitate tumor dissemination at several key steps of metastasis, including adherence of tumor cells to endothelium, extravasation from blood vessels, metastatic colonization, angiogenesis, proliferation, and protection from the host response via activation of key survival pathways such as phosphatidylinositol-3 kinase and Akt. It is interesting that many of these roles are reminiscent of their functions in leukocyte and stem cell trafficking. Lastly, we discuss therapeutic applications for chemokine receptor antagonists in cancer therapy.
The evolution of mammalian chemokine genes
[J].
Chemokines
[J].Chemokines are small proteins that control cellular migration. An extensive family of these molecules has been described in mammals containing nearly 50 members. Within this family are four groups, each defined by the different spacing of two N-terminal cysteines, which form disulphide bonds with two other cysteine residues to create the tertiary structure characteristic of chemokines. Recent evidence shows the chemokine family is not unique to mammals, with several members also identified in birds, amphibians and fish, including a primitive vertebrate, the lamprey. Although there is less evidence to define the roles of chemokines in these lower vertebrates, structural similarities allow some predictions to their function, against which further studies are being made. Additionally, some microorganisms (particularly viruses) appear to have copied genes for chemokines, presumably to confuse the immune system of their host. This review aims to bring together the current information concerning identified chemokines throughout vertebrates and microorganisms.
Cutting edge: immature dendritic cells generated from monocytes in the presence of TGF-beta 1 express functional C-C chemokine receptor 6
[J].Although CD34+ progenitor-derived immature dendritic cells (DCs) express CCR6, several recent studies reported that monocyte-derived immature DCs do not do so. We observed that DCs generated from monocytes in the presence of GM-CSF, IL-4, and TGF-beta 1 consistently responded to liver and activation-regulated chemokine (LARC, also known as macrophage inflammatory protein-3 alpha). These immature DCs expressed one class of high-affinity binding sites for LARC, and expressed both CCR6 mRNA and protein. Therefore, LARC-CCR6 interaction presumably also contributes to the regulation of trafficking of monocyte-derived DCs, and utilization of TGF-beta can potentially provide a ready source of CCR6+ monocyte-derived DCs for therapeutic purposes.
CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells
[J].Dendritic cells initiate immune responses by ferrying antigen from the tissues to the lymphoid organs for presentation to lymphocytes. Little is known about the molecular mechanisms underlying this migratory behavior. We have identified a chemokine receptor which appears to be selectively expressed in human dendritic cells derived from CD34+ cord blood precursors, but not in dendritic cells derived from peripheral blood monocytes. When stably expressed as a recombinant protein in a variety of host cell backgrounds, the receptor shows a strong interaction with only one chemokine among 25 tested: the recently reported CC chemokine macrophage inflammatory protein 3α. Thus, we have designated this receptor as the CC chemokine receptor 6. The cloning and characterization of a dendritic cell CC chemokine receptor suggests a role for chemokines in the control of the migration of dendritic cells and the regulation of dendritic cell function in immunity and infection.
Regulation of CCR6 chemokine receptor expression and responsiveness to macrophage inflammatory protein-3alpha/CCL20 in human B cells
[J].The regulation of CCR6 (chemokine receptor 6) expression during B-cell ontogeny and antigen-driven B-cell differentiation was analyzed. None of the CD34(+)Lin(-) hematopoietic stem cell progenitors or the CD34(+)CD19(+) (pro-B) or the CD19(+)CD10(+) (pre-B/immature B cells) B-cell progenitors expressed CCR6. CCR6 is acquired when CD10 is lost and B-cell progeny matures, entering into the surface immunoglobulin D(+) (sIgD(+)) mature B-cell pool. CCR6 is expressed by all bone marrow-, umbilical cord blood-, and peripheral blood-derived naive and/or memory B cells but is absent from germinal center (GC) B cells of secondary lymphoid organs. CCR6 is down-regulated after B-cell antigen receptor triggering and remains absent during differentiation into immunoglobulin-secreting plasma cells, whereas it is reacquired at the stage of post-GC memory B cells. Thus, within the B-cell compartment, CCR6 expression is restricted to functionally mature cells capable of responding to antigen challenge. In transmigration chemotactic assays, macrophage inflammatory protein (MIP)-3alpha/CC chemokine ligand 20 (CCL20) induced vigorous migration of B cells with differential chemotactic preference toward sIgD(-) memory B cells. These data suggest that restricted patterns of CCR6 expression and MIP-3alpha/CCL20 responsiveness are integral parts of the process of B-lineage maturation and antigen-driven B-cell differentiation.
CCR6 regulates the migration of inflammatory and regulatory T cells
[J].Th17 and regulatory T (Treg) cells play opposite roles in autoimmune diseases. However, the mechanisms underlying their proper migration to inflammatory tissues are unclear. In this study, we report that these two T cell subsets both express CCR6. CCR6 expression in Th17 cells is regulated by TGF-beta and requires two nuclear receptors, RORalpha and RORgamma. Th17 cells also express the CCR6 ligand CCL20, which is induced synergistically by TGF-beta and IL-6, which requires STAT3, RORgamma and IL-21. Th17 cells, by producing CCL20, promote migration of Th17 and Treg cells in vitro in a CCR6-dependent manner. Lack of CCR6 in Th17 cells reduces the severity of experimental autoimmune encephalomyelitis and Th17 and Treg recruitment into inflammatory tissues. Similarly, CCR6 on Treg cells is also important for their recruitment into inflammatory tissues. Our data indicate an important role of CCR6 in Treg and Th17 cell migration.
CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3alpha
[J].CC-chemokine receptor (CCR) 6 is the only known receptor for macrophage inflammatory protein (MIP)-3alpha, a CC chemokine chemotactic for lymphocytes and dendritic cells. Using anti-serum that we raised against the N-terminal residues of CCR6, we have characterized the surface expression of CCR6 on peripheral blood leukocytes and we have correlated CCR6 expression with responses to MIP-3alpha. We found that CCR6 was expressed only on memory T cells, including most alpha4beta7 memory cells and cutaneous lymphocyte-associated Ag-expressing cells, and on B cells. Accordingly, chemotaxis of T cells to MIP-3alpha was limited to memory cells. Moreover, calcium signals on T cells in response to MIP-3a were confined to CCR6-expressing cells, consistent with CCR6 being the only MIP-3alpha receptor on peripheral blood T cells. Unlike many CC chemokines, MIP-3alpha produced a calcium signal on freshly isolated T cells, and CCR6 expression was not increased by up to 5 days of treatment with IL-2 or by cross-linking CD3. Despite their surface expression of CCR6, freshly isolated B cells did not respond to MIP-3alpha. In addition to staining peripheral blood leukocytes, our anti-serum detected CCR6 on CD34+ bone marrow cell-derived dendritic cells. Our data are the first to analyze surface expression of CCR6, demonstrating receptor expression on differentiated, resting memory T cells, indicating differences in receptor signaling on T cells and B cells and suggesting that CCR6 and MIP-3alpha may play a role in the physiology of resting memory T cells and in the interactions of memory T cells, B cells, and dendritic cells.
CCR6 ligands inhibit HIV by inducing APOBEC3G
[J].We have identified a post-entry CCR6-dependent mechanism of inhibition of HIV occurring at an early stage of infection mediated by the induction of the host restriction factor apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G (APOBEC3G). We observed induction of APOBEC3G expression only in CCR6(+) cells but not in cells treated with the G inhibitory (Gi) pathway inhibitor pertussis toxin. CCR6 is highly expressed on peripheral blood CD4(+)CCR5(+) memory T cells and by 2 populations of CD4(+) T cells within the gut, alpha4beta7(+) and T helper type 17, that have been implicated in cell-to-cell spread of HIV and enhanced restoration of CD4(+) T cells within gut-associated lymphoid tissue, respectively. This novel CCR6-mediated mechanism of inhibition allows the identification of pathways that induce intrinsic immunity to HIV, which could be useful in devising novel therapeutics that selectively target CCR6(+) cells.
Molecular cloning, characterization and expression analysis of a C-type lectin (Ec-CTL) in orange-spotted grouper, Epinephelus coioides
[J].
Characterization of C-C chemokine receptor subfamily in teleost fish
[J].Chemokines and their receptors play important roles in nervous and immune systems. Little information, however, exists concerning this gene family in teleost fish. In the present study, 17 C-C chemokine receptors genes were identified from Danio rerio, 9 from Gasterosteus aculeatus, 10 from Oryzias latipes, 8 from Takifugu rubripes and 5 from Tetraodon nigroviridis. Phylogenetic analysis showed that the orthologs to mammalian CCR6, 7, 8, 9 and CCRL1 receptors were evident in zebrafish, but the clear orthologs to mammalian CCR1, 2, 3, 4, 5 and 10 were not found in zebrafish. The gene structure of zebrafish CCR (zfCCR) was further analyzed. The open reading frame of zfCCR3-1, zfCCR3-3, zfCCR6-1, zfCCR6-2, zfCCR8-2 contain one exon, and two exons were identified for zfCCR2-1, zfCCR2-2, zfCCR4 and zfCCRL1-1, three exons for zfCCR3-2, zfCCR5 and zfCCR7, four exons for zfCCR8-1 and zfCCR9-1. The expression analyses showed that in zebrafish, most C-C chemokine receptor genes were expressed in fertilized eggs and oocytes, and all the receptor genes were expressed in larval stages. The zfCCR2-2, zfCCR3-1, zfCCR4 and zfCCR6-2 genes were expressed in all normal organs examined, whereas not for zfCCR2-1, zfCCR3-3, zfCCR6-1, zfCCR8-1, zfCCR9-2 and zfCCRL1-2. The expression of zfCCR3-2, zfCCR5, zfCCR7, zfCCR9-1 and zfCCRL1-1 were detected in the majority organs, and zfCCR8-2 and zfCCR8-3 detected only in brain. The differential expression pattern of different paralogues in organs may indicate their difference in function, which requires further investigation.
Chemokine receptors in Atlantic salmon
[J].Teleost sequence data have revealed that many immune genes have evolved differently when compared to other vertebrates. Thus, each gene family needs functional studies to define the biological role of individual members within major species groups. Chemokine receptors, being excellent markers for various leukocyte subpopulations, are one such example where studies are needed to decipher individual gene function. The unique salmonid whole genome duplication that occurred approximately 95 million years ago has provided salmonids with many additional duplicates further adding to the complexity and diversity. Here we have performed a systematic study of these receptors in Atlantic salmon with particular focus on potential inflammatory receptors. Using the preliminary salmon genome data we identified 48 chemokine or chemokine-like receptors including orthologues to the ten receptors previously published in trout. We found expressed support for 40 of the bona fide salmon receptors. Eighteen of the chemokine receptors are duplicated, and when tested against a diploid sister group the majority were shown to be remnants of the 4R whole genome duplication with subsequent high sequence identity. The salmon chemokine receptor repertoire of 40 expressed bona fide genes is comparably larger than that found in humans with 23 receptors. Diversification has been a major driving force for these duplicate genes with the main variability residing in ligand binding and signalling domains. Copyright © 2014 Elsevier Ltd. All rights reserved.
Molecular characterization and expression analysis of four fish-specific CC chemokine receptors CCR4La, CCR4Lc1, CCR4Lc2 and CCR11 in rainbow trout (Oncorhynchus mykiss)
[J].
Chemokines and their receptors in the central nervous system
[J].Chemokines are a family of proteins associated with the trafficking of leukocytes in physiological immune surveillance and inflammatory cell recruitment in host defence. They are classified into four classes based on the positions of key cystiene residues: C, CC, CXC, and CX3C. Chemokines act through both specific and shared receptors that all belong to the superfamily of G-protein-coupled receptors. Besides their well-established role in the immune system, several recent reports have demonstrated that these proteins also play a role in the central nervous system (CNS). In the CNS, chemokines are constitutively expressed by microglial cells, astrocytes, and neurons, and their expression can be increased after induction with inflammatory mediators. Constitutive expression of chemokines and chemokine receptors has been observed in both developing and adult brains, and the role played by these proteins in the normal brain is the object of intense study by many research groups. Chemokines are involved in brain development and in the maintenance of normal brain homeostasis; these proteins play a role in the migration, differentiation, and proliferation of glial and neuronal cells. The chemokine stromal cell-derived factor 1 and its receptor, CXCR4, are essential for life during development, and this ligand-receptor pair has been shown to have a fundamental role in neuron migration during cerebellar formation. Chemokine and chemokine receptor expression can be increased by inflammatory mediators, and this has in turn been associated with several acute and chronic inflammatory conditions. In the CNS, chemokines play an essential role in neuroinflammation as mediators of leukocyte infiltration. Their overexpression has been implicated in different neurological disorders, such as multiple sclerosis, trauma, stroke, Alzheimer's disease, tumor progression, and acquired immunodeficiency syndrome-associated dementia. An emerging area of interest for chemokine action is represented by the communication between the neuroendocrine and the immune system. Chemokines have hormone-like actions, specifically regulating the key host physiopathological responses of fever and appetite. It is now evident that chemokines and their receptors represent a plurifunctional family of proteins whose actions on the CNS are not restricted to neuroinflammation. These molecules constitute crucial regulators of cellular communication in physiological and developmental processes.Copyright 2001 Academic Press.
The role of CCL20-CCR6 axis in ovarian cancer metastasis
[J].
The CC chemokine CCL20 and its receptor CCR6
[J].
The CCL20-CCR6 axis in cancer progression
[J].Chemokines, which are basic proteins that exert their effects via G protein-coupled receptors and a subset of the cytokine family, are mediators deeply involved in leukocyte migration during an inflammatory reaction. Chemokine (C-C motif) ligand 20 (CCL20), also known as macrophage inflammatory protein (MIP)-3α, liver activation regulated chemokine (LARC), and Exodus-1, is a small protein that is physiologically expressed in the liver, colon, and skin, is involved in tissue inflammation and homeostasis, and has a specific receptor C-C chemokine receptor 6 (CCR6). The CCL20-CCR6 axis has long been known to be involved in inflammatory and infectious diseases, such as rheumatoid arthritis and human immunodeficiency virus infections. Recently, however, reports have shown that the CCL20-CCR6 axis is associated with several cancers, including hepatocellular carcinoma, colorectal cancer, breast cancer, pancreatic cancer, cervical cancer, and kidney cancer. The CCL20-CCR6 axis promotes cancer progression directly by enhancing migration and proliferation of cancer cells and indirectly by remodeling the tumor microenvironment through immune cell control. The present article reviewed the role of the CCL20-CCR6 axis in cancer progression and its potential as a therapeutic target.
CCR6 as a possible therapeutic target in psoriasis
[J].Psoriasis is a common, chronic autoimmune disease of the skin. Despite a number of effective treatments, new therapies are needed with enhanced efficacy, safety and convenience. Chemokine receptors are GPCRs that control leukocyte trafficking, and like other GPCRs, are good potential drug targets. The chemokine receptor CCR6 is expressed on the T(H)17 subset of CD4(+) T cells, which produces IL-17A/F, IL-22, TNF-alpha and other cytokines, and which has been implicated in the pathogenesis of psoriasis. CCR6 and its ligand, CCL20/MIP-3alpha, are highly expressed in psoriatic skin and CCR6 is necessary for the pathology induced in a mouse model of psoriasis-like inflammation.This review summarizes the evidence for the importance of the IL-23/T(H)17 axis, and in particular CCR6 and CCL20 in psoriasis, dating from 2000 to the present, and discusses the possibility of inhibiting CCR6 as a treatment for the disease.The review informs the reader of the current thinking on the mechanisms of inflammation in psoriasis and the possible roles for CCR6 (and CCL20) in disease pathogenesis.We conclude that CCR6 should be investigated as a potential therapeutic target in psoriasis.
The CC and CXC chemokine receptors in turbot (Scophthalmus maximus L.) and their response to Aeromonas salmonicida infection
[J].
Characterization and expression analysis of two novel CCR6 chemokine receptors and their three potential ligands CCL20Ls of grouper (Epinephelus coioides) post Cryptocaryon irritans infection
[J].
CCR6 functions as a new coreceptor for limited primary human and simian immunodeficiency viruses
[J].
CCR6/CCL20 chemokine axis in human immunodeficiency virus immunity and pathogenesis
[J].Recent studies in human immunodeficiency virus (HIV) have garnered interest for the role of CC chemokine receptor 6 (CCR6) and its known ligands, CC chemokine ligand 20 (CCL20) and human β-defensins, in viral entry, dissemination and antiviral immunity. Several studies have suggested that CCR6 may also act as a weak co-receptor of HIV entry, in addition to the canonical CXC chemokine receptor 4 (CXCR4) and CCR5. However, the pathogenic significance has yet to be demonstrated as the observations for preferential infection of CD4+CCR6+ over CD4+CCR6- T cells appear to be independent of CCR6 expression. This indicates means for preferential infection other than CCR6 co-receptor use. Attention has also turned to the inadvertent role of the CCR6/CCL20 axis in attracting key immune cells, including TH17 cells and dendritic cells, to sites of infection and propagating the virus to other sites of the body. This review article will summarize the latest evidence that the CCR6/CCL20 chemokine axis is playing an important role in HIV pathogenesis and immunity. Further work with in vivo studies is needed to establish the biological and, hence, therapeutic significance of these findings.
Chemokine transcription in rainbow trout (Oncorhynchus mykiss) is differently modulated in response to viral hemorrhagic septicaemia virus (VHSV) or infectious pancreatic necrosis virus (IPNV)
[J].
Specific regulation of the chemokine response to viral hemorrhagic septicemia virus at the entry site
[J].The fin bases constitute the main portal of rhabdovirus entry into rainbow trout (Oncorhynchus mykiss), and replication in this first site strongly conditions the outcome of the infection. In this context, we studied the chemokine response elicited in this area in response to viral hemorrhagic septicemia virus (VHSV), a rhabdovirus. Among all the rainbow trout chemokine genes studied, only the transcription levels of CK10 and CK12 were significantly upregulated in response to VHSV. As the virus had previously been shown to elicit a much stronger chemokine response in internal organs, we compared the effect of VHSV on the gills, another mucosal site which does not constitute the main site of viral entry or rhabdoviral replication. In this case, a significantly stronger chemokine response was triggered, with CK1, CK3, CK9, and CK11 being upregulated in response to VHSV and CK10 and CK12 being down-modulated by the virus. We then conducted further experiments to understand how these different chemokine responses of mucosal tissues could correlate with their capacity to support VHSV replication. No viral replication was detected in the gills, while at the fin bases, only the skin and the muscle were actively supporting viral replication. Within the skin, viral replication took place in the dermis, while viral replication was blocked within epidermal cells at some point before protein translation. The different susceptibilities of the different skin layers to VHSV correlated with the effect that VHSV has on their capacity to secrete chemotactic factors. Altogether, these results suggest a VHSV interference mechanism on the early chemokine response at its active replication sites within mucosal tissues, a possible key process that may facilitate viral entry.
DNA vaccination against a fish rhabdovirus promotes an early chemokine-related recruitment of B cells to the muscle
[J].In fish, intramuscular (i.m) injection of plasmid DNA encoding viral proteins has proved a highly effective vaccination strategy against some viral pathogens. The efficacy of DNA vaccination in teleost fish is based on the high level of viral antigen expression in muscle cells inducing a strong and long-lasting protection. However, the mechanisms through which this protection is established and effectuated in fish are still not fully understood. Moreover, similarities to mammalian models cannot be established since DNA vaccination in mammals usually induces much weaker responses. In this work, we have focused on the characterization of the immune cells that infiltrate the muscle at the site of DNA injection in vaccinated fish and the chemokines and chemokine receptors that may be involved in their infiltration. We have demonstrated through diverse techniques that B lymphocytes, both IgM⁺ and IgT⁺ cells, represented a major infiltrating cell type in fish vaccinated with a viral haemorrhagic septicaemia virus (VHSV) glycoprotein-encoding DNA vaccine, whereas in control fish injected with an oil adjuvant mainly granulocyte/monocyte-type cells were attracted. Among twelve chemokine genes studied, only CXCL11_L1, CK5B and CK6 mRNA levels were up-regulated in DNA vaccinated fish compared to fish injected with the corresponding vector backbone. Furthermore, the transcription of CXCR3B, a possible receptor for CXCL11_L1 was also significantly up-regulated in vaccinated fish. Finally, experiments performed with recombinant trout CK5B and CK6 and chemokine expression plasmids revealed that these chemokines have chemotactic capacities which might explain the recruitment of B cells to the site of DNA injection. Altogether, our results reveal that there is an early chemokine-related B cell recruitment triggered by i.m. DNA vaccination against VHSV which might play an important role in the initial phase of the immune response.Copyright © 2013 Elsevier Ltd. All rights reserved.
Expression and subcellular analyses of CCR8a/b genes with the identification of response to SGIV viral infect in orange-spotted grouper (Epinephelus coioides)
[J].
赤点石斑鱼抗神经坏死病的基因组选择评估
[J].赤点石斑鱼(Epinephelus akaara)是中国东南沿海地区一种重要的海水养殖鱼类,多年来其苗种生产深受神经坏死病的困扰,严重制约了其人工养殖业的发展。本研究采集230尾赤点石斑鱼神经坏死病毒(RGNNV)易感(染病死亡)和230尾抗性(最终健康存活)赤点石斑鱼苗进行基因组重测序,分析获得5 412 683个单核苷酸多态性(SNPs)位点的基因型,并以之对抗病性状进行了遗传评估和基因组选择研究,获得的估计遗传力均值为0.566 2,预测基因组估计育种值(GEBV)均值为0.154 3。随机选择不同数量标记对基因组选择准确性影响进行评估,结果表明,采用5 000个(5 k)标记进行赤点石斑鱼抗神经坏死病性状遗传评估就可以获得比较理想的效果。本研究为开展赤点石斑鱼抗神经坏死病基因组选择育种实践提供了有用的理论参考。
神经坏死病毒疫苗免疫后3种免疫相关基因在斜带石斑鱼 (Epinephelus coioides)组织中的表达研究
[J].感染神经坏死病毒 (Nervous necrosis virus, NNV) 会引起斜带石斑鱼 (Epinephelus coioides) 苗种大量死亡。病毒样颗粒 (Virus-like particles, VLPs)不含病毒基因组,被认为是一种最安全、有效的能够预防病毒病的疫苗。本研究以养殖的斜带石斑鱼为研究对象,分析了3种免疫相关基因(IFN、TNF-α和IRF3)在9种组织(心、肝、脾、肾、肠、鳃、血、眼和脑)中的表达量,并通过肌肉注射疫苗,检测这3种免疫相关基因在主要免疫组织(肝、脾和肾)中的表达变化情况。结果显示,IFN、TNF-α和IRF3基因在斜带石斑鱼的9种组织中都有表达,IFN、TNF-α和IRF3基因分别在脑、鳃和血中的表达量最高。单独疫苗免疫后,肝脏中IFN、TNF-α和IRF3基因,脾脏中IFN基因以及肾脏中IFN基因的相对表达量并没有提高,但注射添加CpG ODN(含CpG基序的寡核苷酸)佐剂的疫苗后,这些基因的相对表达量均出现上调。单独疫苗免疫能够提高其它组织中IFN、TNF-α和IRF3基因的表达值,但其最高表达量均小于相对应的佐剂+疫苗组,表明CpG寡核苷酸基序能够提高疫苗的免疫原性和机体的免疫反应能力,从而使疫苗发挥最大的免疫作用。